

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosylhomocysteinase (Mus musculus) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50373101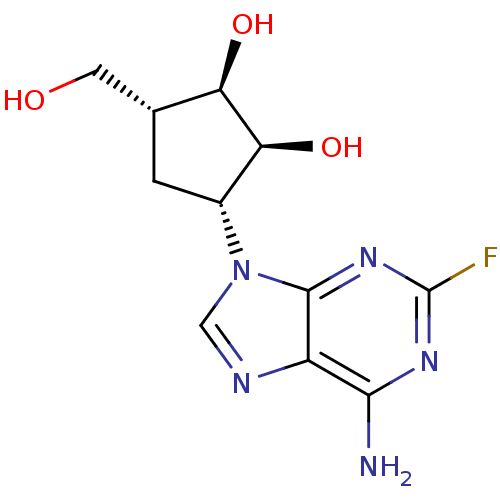 (CHEMBL405186) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SAHH | Bioorg Med Chem 16: 3809-15 (2008) Article DOI: 10.1016/j.bmc.2008.01.046 BindingDB Entry DOI: 10.7270/Q2RJ4KB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50373100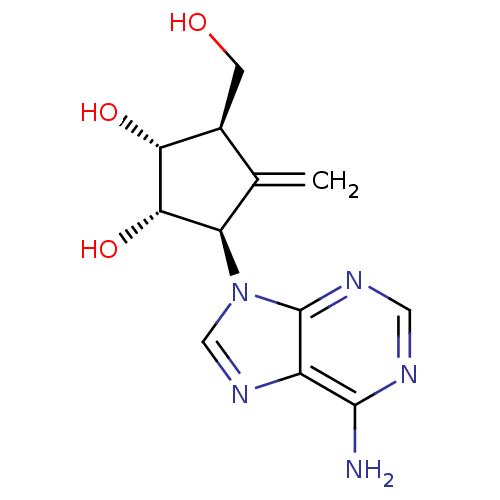 (CHEMBL262063) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SAHH | Bioorg Med Chem 16: 3809-15 (2008) Article DOI: 10.1016/j.bmc.2008.01.046 BindingDB Entry DOI: 10.7270/Q2RJ4KB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50135288 ((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-cyclopentane-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against P. falciparum S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50135288 ((1S,2R,3S,4R)-4-(6-Amino-purin-9-yl)-cyclopentane-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SAHH | Bioorg Med Chem 16: 3809-15 (2008) Article DOI: 10.1016/j.bmc.2008.01.046 BindingDB Entry DOI: 10.7270/Q2RJ4KB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50373102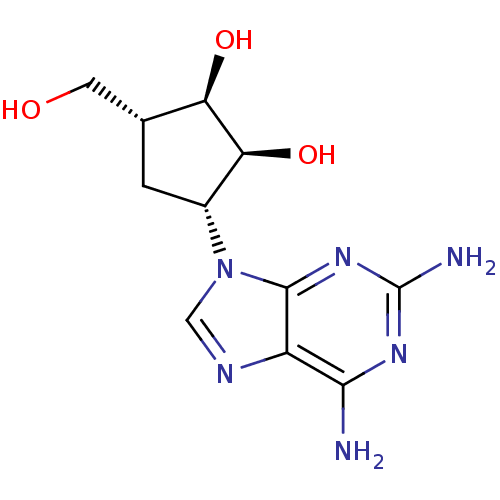 (CHEMBL261619) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SAHH | Bioorg Med Chem 16: 3809-15 (2008) Article DOI: 10.1016/j.bmc.2008.01.046 BindingDB Entry DOI: 10.7270/Q2RJ4KB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50135289 ((1S,2R,3S,4R)-4-(6-Amino-2-fluoro-purin-9-yl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against P. falciparum S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 13: 3963-5 (2003) BindingDB Entry DOI: 10.7270/Q2125S2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50135289 ((1S,2R,3S,4R)-4-(6-Amino-2-fluoro-purin-9-yl)-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SAHH | Bioorg Med Chem 16: 3809-15 (2008) Article DOI: 10.1016/j.bmc.2008.01.046 BindingDB Entry DOI: 10.7270/Q2RJ4KB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Plasmodium falciparum 3D7) | BDBM50088426 ((1R,2S,3R,5R)-3-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum SAHH | Bioorg Med Chem 16: 3809-15 (2008) Article DOI: 10.1016/j.bmc.2008.01.046 BindingDB Entry DOI: 10.7270/Q2RJ4KB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023889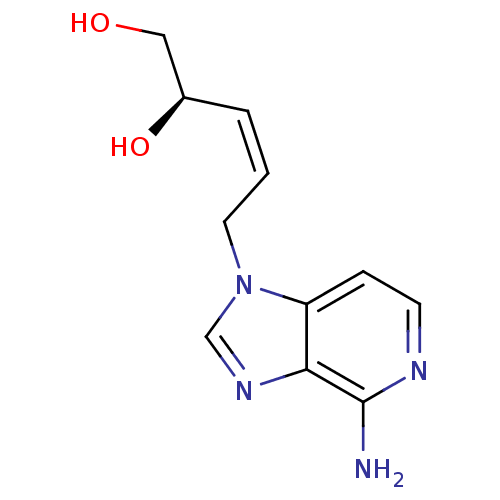 (5-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-pent-3-ene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023879 (2-[2-(6-Amino-purin-9-yl)-ethylidene]-propane-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023882 (5-(6-Amino-purin-9-yl)-pent-3-ene-1,2-diol | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023886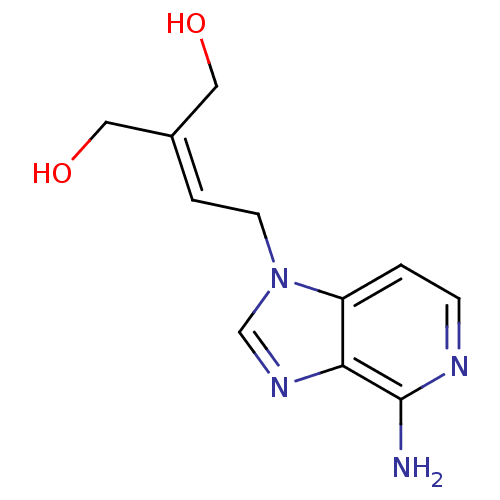 (2-[2-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-ethylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50011148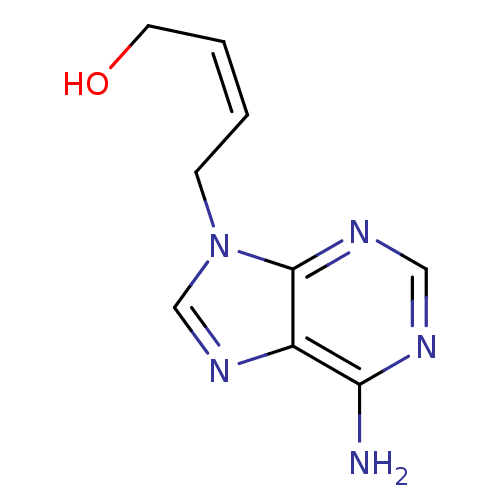 ((Z)-4-(6-Amino-purin-9-yl)-but-2-en-1-ol | 4-(6-Am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023887 (4-(6-Amino-purin-9-yl)-2-methyl-but-2-en-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023877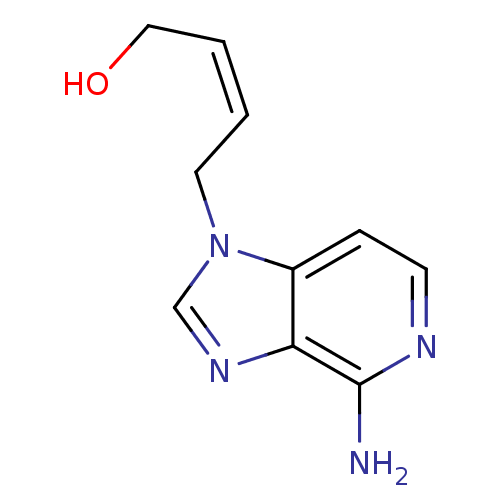 (4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-en-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023885 (4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-yn-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50011180 (4-(6-Amino-purin-9-yl)-but-2-en-1-ol | CHEMBL49917) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50408389 (CHEMBL2051757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Auburn University Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound against purified S-Adenosylhomocysteine hydrolase isolated from murine L929 cells | J Med Chem 40: 622-4 (1997) Article DOI: 10.1021/jm9605039 BindingDB Entry DOI: 10.7270/Q2FF3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50408388 (CHEMBL2051967) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Auburn University Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound against purified S-Adenosylhomocysteine hydrolase isolated from murine L929 cells | J Med Chem 40: 622-4 (1997) Article DOI: 10.1021/jm9605039 BindingDB Entry DOI: 10.7270/Q2FF3RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||