 Found 70 hits of ic50 for UniProtKB: O08786
Found 70 hits of ic50 for UniProtKB: O08786 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284146
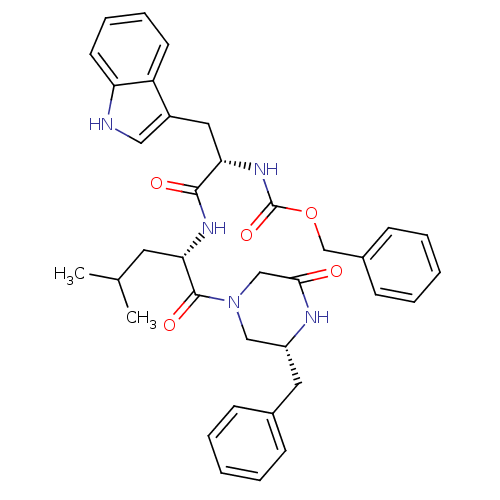
(CHEMBL355608 | [(S)-1-[(S)-1-((R)-3-Benzyl-5-oxo-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(=O)N1C[C@@H](Cc2ccccc2)NC(=O)C1 Show InChI InChI=1S/C36H41N5O5/c1-24(2)17-32(35(44)41-21-28(38-33(42)22-41)18-25-11-5-3-6-12-25)39-34(43)31(19-27-20-37-30-16-10-9-15-29(27)30)40-36(45)46-23-26-13-7-4-8-14-26/h3-16,20,24,28,31-32,37H,17-19,21-23H2,1-2H3,(H,38,42)(H,39,43)(H,40,45)/t28-,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284148
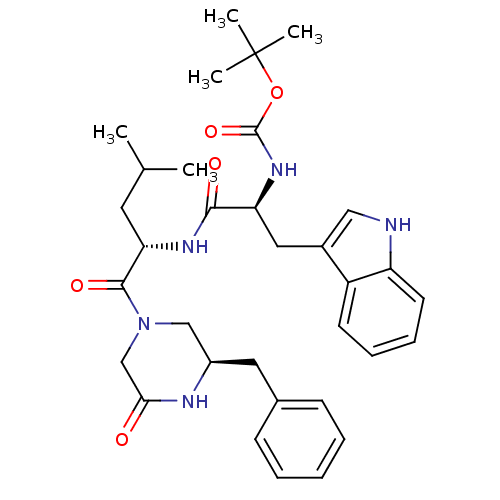
(CHEMBL171310 | [(S)-1-[(S)-1-((R)-3-Benzyl-5-oxo-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N1C[C@@H](Cc2ccccc2)NC(=O)C1 Show InChI InChI=1S/C33H43N5O5/c1-21(2)15-28(31(41)38-19-24(35-29(39)20-38)16-22-11-7-6-8-12-22)36-30(40)27(37-32(42)43-33(3,4)5)17-23-18-34-26-14-10-9-13-25(23)26/h6-14,18,21,24,27-28,34H,15-17,19-20H2,1-5H3,(H,35,39)(H,36,40)(H,37,42)/t24-,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284156

(CHEMBL169174 | [(S)-1-[(S)-1-((S)-3-Benzyl-5-oxo-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(=O)N1C[C@H](Cc2ccccc2)NC(=O)C1 Show InChI InChI=1S/C36H41N5O5/c1-24(2)17-32(35(44)41-21-28(38-33(42)22-41)18-25-11-5-3-6-12-25)39-34(43)31(19-27-20-37-30-16-10-9-15-29(27)30)40-36(45)46-23-26-13-7-4-8-14-26/h3-16,20,24,28,31-32,37H,17-19,21-23H2,1-2H3,(H,38,42)(H,39,43)(H,40,45)/t28-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287255
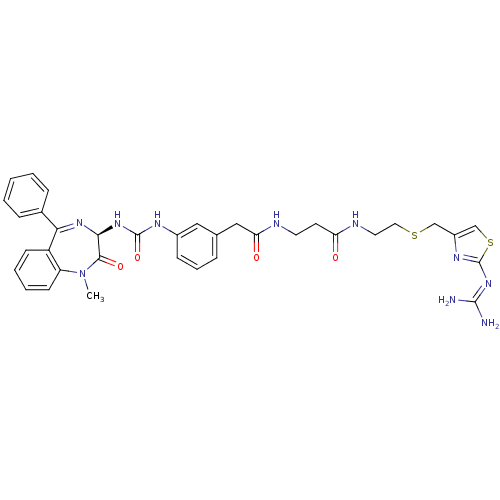
(CHEMBL30821 | N-[2-(2-Guanidino-thiazol-4-ylmethyl...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6@@H](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](\[#7])-[#7])n3)c2)-[#6]-1=O)-c1ccccc1 |c:9| Show InChI InChI=1S/C35H38N10O4S2/c1-45-27-13-6-5-12-26(27)30(23-9-3-2-4-10-23)42-31(32(45)48)43-34(49)40-24-11-7-8-22(18-24)19-29(47)38-15-14-28(46)39-16-17-50-20-25-21-51-35(41-25)44-33(36)37/h2-13,18,21,31H,14-17,19-20H2,1H3,(H,38,47)(H,39,46)(H2,40,43,49)(H4,36,37,41,44)/t31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287253
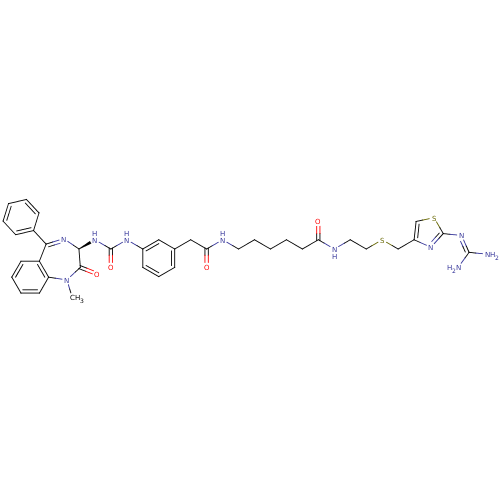
(6-(2-{3-[3-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydr...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6@@H](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](/[#7])-[#7])n3)c2)-[#6]-1=O)-c1ccccc1 |c:9| Show InChI InChI=1S/C38H44N10O4S2/c1-48-30-16-8-7-15-29(30)33(26-12-4-2-5-13-26)45-34(35(48)51)46-37(52)43-27-14-10-11-25(21-27)22-32(50)41-18-9-3-6-17-31(49)42-19-20-53-23-28-24-54-38(44-28)47-36(39)40/h2,4-5,7-8,10-16,21,24,34H,3,6,9,17-20,22-23H2,1H3,(H,41,50)(H,42,49)(H2,43,46,52)(H4,39,40,44,47)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287234
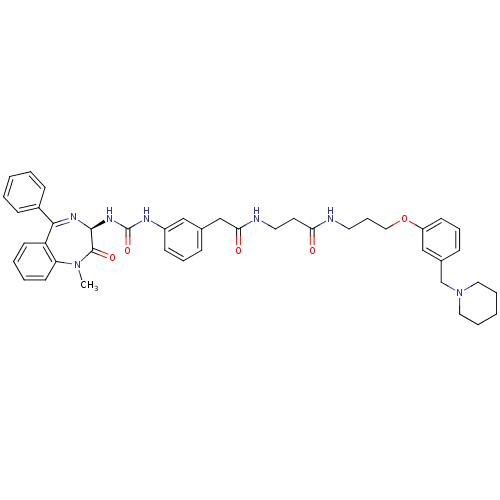
(3-(2-{3-[3-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydr...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CC(=O)NCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C43H49N7O5/c1-49-37-20-7-6-19-36(37)40(33-15-4-2-5-16-33)47-41(42(49)53)48-43(54)46-34-17-10-13-31(27-34)29-39(52)45-23-21-38(51)44-22-12-26-55-35-18-11-14-32(28-35)30-50-24-8-3-9-25-50/h2,4-7,10-11,13-20,27-28,41H,3,8-9,12,21-26,29-30H2,1H3,(H,44,51)(H,45,52)(H2,46,48,54)/t41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284147
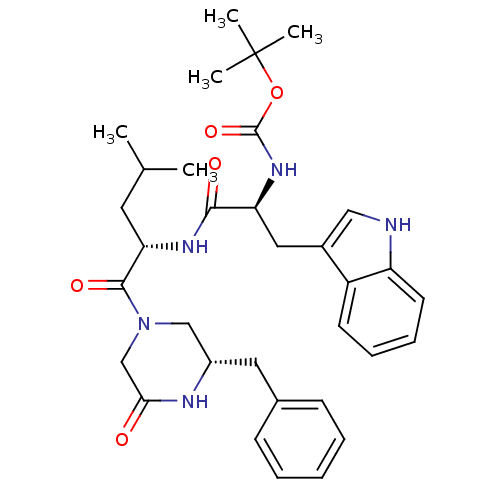
(CHEMBL354363 | [(S)-1-[(S)-1-((S)-3-Benzyl-5-oxo-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N1C[C@H](Cc2ccccc2)NC(=O)C1 Show InChI InChI=1S/C33H43N5O5/c1-21(2)15-28(31(41)38-19-24(35-29(39)20-38)16-22-11-7-6-8-12-22)36-30(40)27(37-32(42)43-33(3,4)5)17-23-18-34-26-14-10-9-13-25(23)26/h6-14,18,21,24,27-28,34H,15-17,19-20H2,1-5H3,(H,35,39)(H,36,40)(H,37,42)/t24-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287265
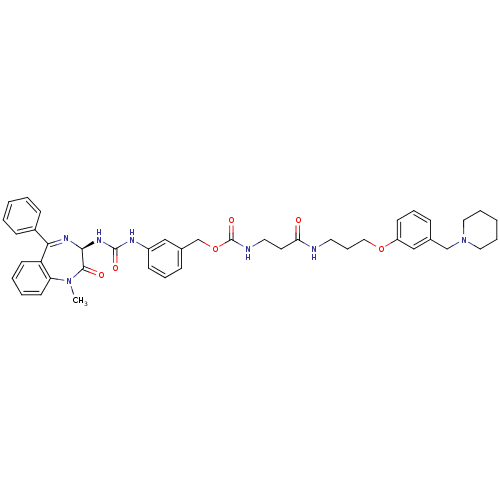
(CHEMBL406844 | {2-[3-(3-Piperidin-1-ylmethyl-pheno...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C43H49N7O6/c1-49-37-20-7-6-19-36(37)39(33-15-4-2-5-16-33)47-40(41(49)52)48-42(53)46-34-17-10-14-32(27-34)30-56-43(54)45-23-21-38(51)44-22-12-26-55-35-18-11-13-31(28-35)29-50-24-8-3-9-25-50/h2,4-7,10-11,13-20,27-28,40H,3,8-9,12,21-26,29-30H2,1H3,(H,44,51)(H,45,54)(H2,46,48,53)/t40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287250
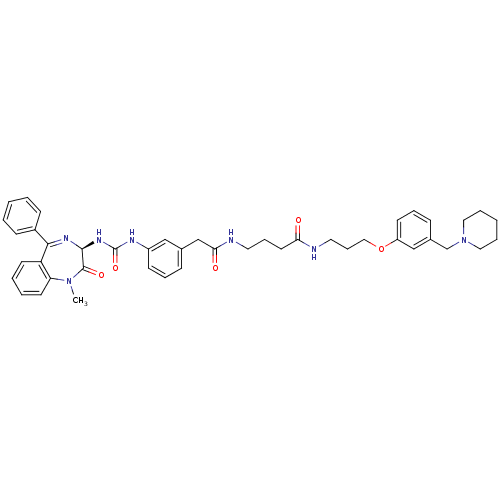
(4-(2-{3-[3-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydr...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CC(=O)NCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C44H51N7O5/c1-50-38-21-7-6-20-37(38)41(34-16-4-2-5-17-34)48-42(43(50)54)49-44(55)47-35-18-10-14-32(28-35)30-40(53)46-23-12-22-39(52)45-24-13-27-56-36-19-11-15-33(29-36)31-51-25-8-3-9-26-51/h2,4-7,10-11,14-21,28-29,42H,3,8-9,12-13,22-27,30-31H2,1H3,(H,45,52)(H,46,53)(H2,47,49,55)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287251

(CHEMBL262125 | N-[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6@@H](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](\[#7])-[#7])n3)c2)-[#6]-1=O)-c1ccccc1 |c:9| Show InChI InChI=1S/C36H40N10O4S2/c1-46-28-14-6-5-13-27(28)31(24-10-3-2-4-11-24)43-32(33(46)49)44-35(50)41-25-12-7-9-23(19-25)20-30(48)39-16-8-15-29(47)40-17-18-51-21-26-22-52-36(42-26)45-34(37)38/h2-7,9-14,19,22,32H,8,15-18,20-21H2,1H3,(H,39,48)(H,40,47)(H2,41,44,50)(H4,37,38,42,45)/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287256

(6-(2-{3-[3-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydr...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CC(=O)NCCCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C46H55N7O5/c1-52-40-23-9-8-22-39(40)43(36-18-5-2-6-19-36)50-44(45(52)56)51-46(57)49-37-20-13-16-34(30-37)32-42(55)48-25-10-3-7-24-41(54)47-26-15-29-58-38-21-14-17-35(31-38)33-53-27-11-4-12-28-53/h2,5-6,8-9,13-14,16-23,30-31,44H,3-4,7,10-12,15,24-29,32-33H2,1H3,(H,47,54)(H,48,55)(H2,49,51,57)/t44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287233
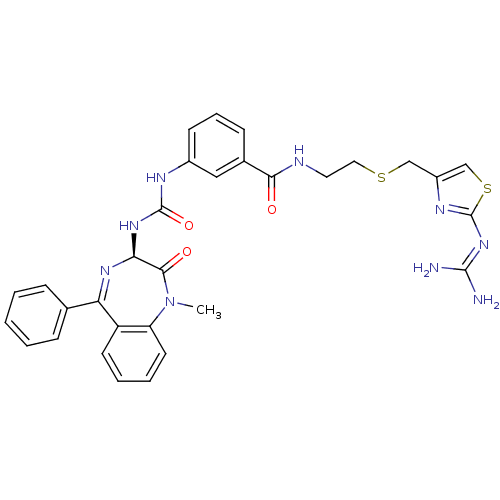
(CHEMBL280980 | N-[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6@@H](-[#7]-[#6](=O)-[#7]-c2cccc(c2)-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c2csc(\[#7]=[#6](\[#7])-[#7])n2)-[#6]-1=O)-c1ccccc1 |c:9| Show InChI InChI=1S/C31H31N9O3S2/c1-40-24-13-6-5-12-23(24)25(19-8-3-2-4-9-19)37-26(28(40)42)38-30(43)35-21-11-7-10-20(16-21)27(41)34-14-15-44-17-22-18-45-31(36-22)39-29(32)33/h2-13,16,18,26H,14-15,17H2,1H3,(H,34,41)(H2,35,38,43)(H4,32,33,36,39)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287259

(CHEMBL286908 | N-[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-c1cccc(-[#7]-[#6](=O)-[#7]-[#6]-2-[#7]=[#6](-c3ccccc3)-c3ccccc3-[#7](-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](\[#7])-[#7])n3)-[#6]-2=O)c1 |t:11| Show InChI InChI=1S/C35H38N10O4S2/c1-22-8-7-11-24(18-22)40-34(49)43-31-32(48)45(27-13-6-5-12-26(27)30(42-31)23-9-3-2-4-10-23)19-29(47)38-15-14-28(46)39-16-17-50-20-25-21-51-35(41-25)44-33(36)37/h2-13,18,21,31H,14-17,19-20H2,1H3,(H,38,47)(H,39,46)(H2,40,43,49)(H4,36,37,41,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287244
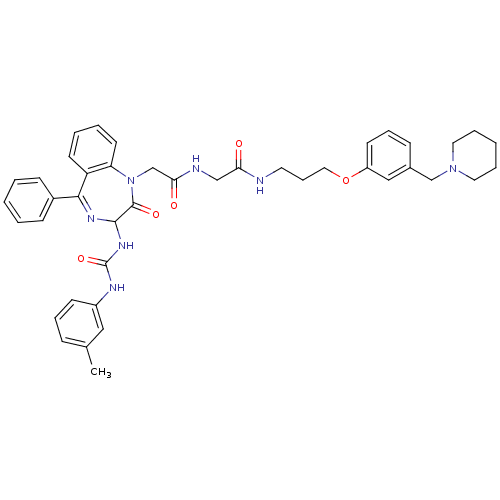
(2-[2-Oxo-5-phenyl-3-(3-m-tolyl-ureido)-2,3-dihydro...)Show SMILES Cc1cccc(NC(=O)NC2N=C(c3ccccc3)c3ccccc3N(CC(=O)NCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)C2=O)c1 |t:11| Show InChI InChI=1S/C42H47N7O5/c1-30-13-10-17-33(25-30)45-42(53)47-40-41(52)49(36-20-7-6-19-35(36)39(46-40)32-15-4-2-5-16-32)29-38(51)44-27-37(50)43-21-12-24-54-34-18-11-14-31(26-34)28-48-22-8-3-9-23-48/h2,4-7,10-11,13-20,25-26,40H,3,8-9,12,21-24,27-29H2,1H3,(H,43,50)(H,44,51)(H2,45,47,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284160

(CHEMBL169178 | [(S)-1-[(S)-1-((S)-3-Benzyl-5-oxo-p...)Show SMILES CCOC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N1C[C@H](Cc2ccccc2)NC(=O)C1 Show InChI InChI=1S/C31H39N5O5/c1-4-41-31(40)35-26(16-22-17-32-25-13-9-8-12-24(22)25)29(38)34-27(14-20(2)3)30(39)36-18-23(33-28(37)19-36)15-21-10-6-5-7-11-21/h5-13,17,20,23,26-27,32H,4,14-16,18-19H2,1-3H3,(H,33,37)(H,34,38)(H,35,40)/t23-,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284161
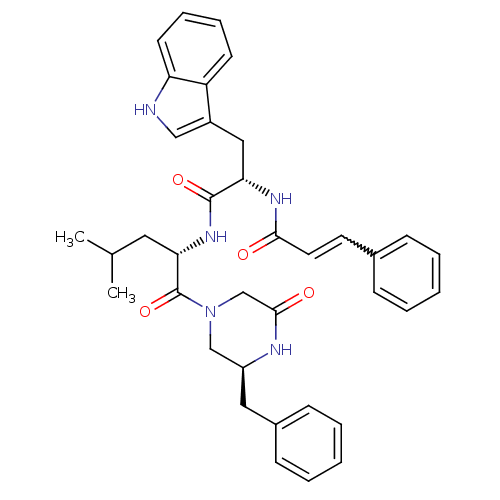
((E)-N-[(S)-1-[(S)-1-((S)-3-Benzyl-5-oxo-piperazine...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)C=Cc1ccccc1)C(=O)N1C[C@H](Cc2ccccc2)NC(=O)C1 |w:23.25| Show InChI InChI=1S/C37H41N5O4/c1-25(2)19-33(37(46)42-23-29(39-35(44)24-42)20-27-13-7-4-8-14-27)41-36(45)32(21-28-22-38-31-16-10-9-15-30(28)31)40-34(43)18-17-26-11-5-3-6-12-26/h3-18,22,25,29,32-33,38H,19-21,23-24H2,1-2H3,(H,39,44)(H,40,43)(H,41,45)/t29-,32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287248
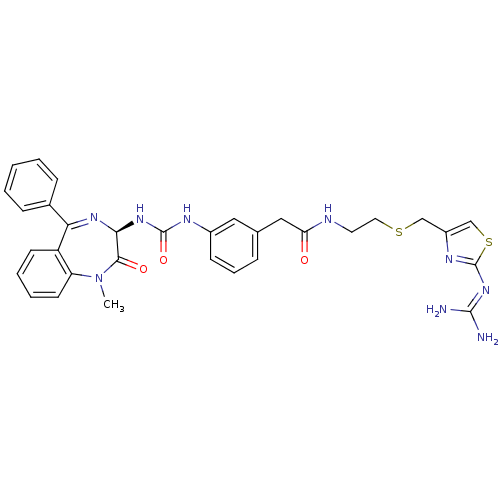
(CHEMBL30430 | N-[2-(2-Guanidino-thiazol-4-ylmethyl...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6@@H](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](/[#7])-[#7])n3)c2)-[#6]-1=O)-c1ccccc1 |c:9| Show InChI InChI=1S/C32H33N9O3S2/c1-41-25-13-6-5-12-24(25)27(21-9-3-2-4-10-21)38-28(29(41)43)39-31(44)36-22-11-7-8-20(16-22)17-26(42)35-14-15-45-18-23-19-46-32(37-23)40-30(33)34/h2-13,16,19,28H,14-15,17-18H2,1H3,(H,35,42)(H2,36,39,44)(H4,33,34,37,40)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284151

(((2S,5S)-1-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N1C[C@H](CCc2ccccc2)NC(=O)[C@@H]1CC(O)=O Show InChI InChI=1S/C36H47N5O7/c1-22(2)17-29(34(46)41-21-25(16-15-23-11-7-6-8-12-23)38-33(45)30(41)19-31(42)43)39-32(44)28(40-35(47)48-36(3,4)5)18-24-20-37-27-14-10-9-13-26(24)27/h6-14,20,22,25,28-30,37H,15-19,21H2,1-5H3,(H,38,45)(H,39,44)(H,40,47)(H,42,43)/t25-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287238
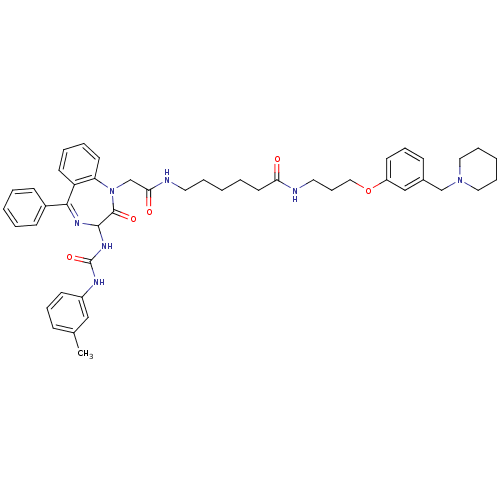
(6-{2-[2-Oxo-5-phenyl-3-(3-m-tolyl-ureido)-2,3-dihy...)Show SMILES Cc1cccc(NC(=O)NC2N=C(c3ccccc3)c3ccccc3N(CC(=O)NCCCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)C2=O)c1 |t:11| Show InChI InChI=1S/C46H55N7O5/c1-34-16-13-20-37(30-34)49-46(57)51-44-45(56)53(40-23-9-8-22-39(40)43(50-44)36-18-5-2-6-19-36)33-42(55)48-25-10-3-7-24-41(54)47-26-15-29-58-38-21-14-17-35(31-38)32-52-27-11-4-12-28-52/h2,5-6,8-9,13-14,16-23,30-31,44H,3-4,7,10-12,15,24-29,32-33H2,1H3,(H,47,54)(H,48,55)(H2,49,51,57) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287236
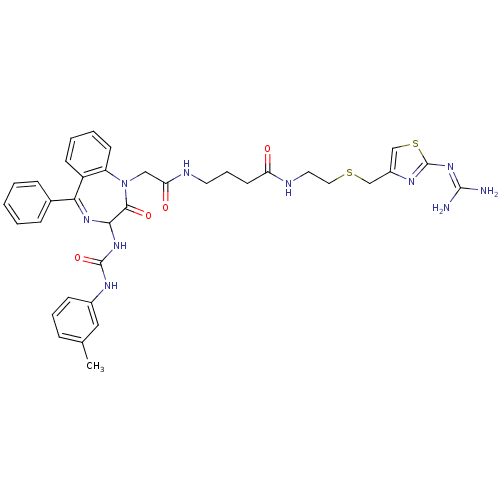
(CHEMBL286907 | N-[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-c1cccc(-[#7]-[#6](=O)-[#7]-[#6]-2-[#7]=[#6](-c3ccccc3)-c3ccccc3-[#7](-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](\[#7])-[#7])n3)-[#6]-2=O)c1 |t:11| Show InChI InChI=1S/C36H40N10O4S2/c1-23-9-7-12-25(19-23)41-35(50)44-32-33(49)46(28-14-6-5-13-27(28)31(43-32)24-10-3-2-4-11-24)20-30(48)39-16-8-15-29(47)40-17-18-51-21-26-22-52-36(42-26)45-34(37)38/h2-7,9-14,19,22,32H,8,15-18,20-21H2,1H3,(H,39,48)(H,40,47)(H2,41,44,50)(H4,37,38,42,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287272

(CHEMBL33412 | [2-(4-{2-[3-(3-Piperidin-1-ylmethyl-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCN3CCN(CC3)C(=O)CNCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C48H59N9O6/c1-54-42-20-7-6-19-41(42)44(38-15-4-2-5-16-38)52-45(46(54)59)53-47(60)51-39-17-10-14-37(31-39)35-63-48(61)50-22-25-55-26-28-57(29-27-55)43(58)33-49-21-12-30-62-40-18-11-13-36(32-40)34-56-23-8-3-9-24-56/h2,4-7,10-11,13-20,31-32,45,49H,3,8-9,12,21-30,33-35H2,1H3,(H,50,61)(H2,51,53,60)/t45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287254
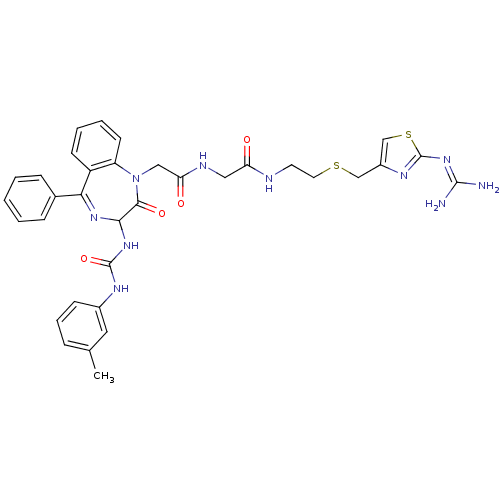
(CHEMBL281082 | N-{[2-(2-Guanidino-thiazol-4-ylmeth...)Show SMILES [#6]-c1cccc(-[#7]-[#6](=O)-[#7]-[#6]-2-[#7]=[#6](-c3ccccc3)-c3ccccc3-[#7](-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](/[#7])-[#7])n3)-[#6]-2=O)c1 |t:11| Show InChI InChI=1S/C34H36N10O4S2/c1-21-8-7-11-23(16-21)39-33(48)42-30-31(47)44(26-13-6-5-12-25(26)29(41-30)22-9-3-2-4-10-22)18-28(46)38-17-27(45)37-14-15-49-19-24-20-50-34(40-24)43-32(35)36/h2-13,16,20,30H,14-15,17-19H2,1H3,(H,37,45)(H,38,46)(H2,39,42,48)(H4,35,36,40,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284158
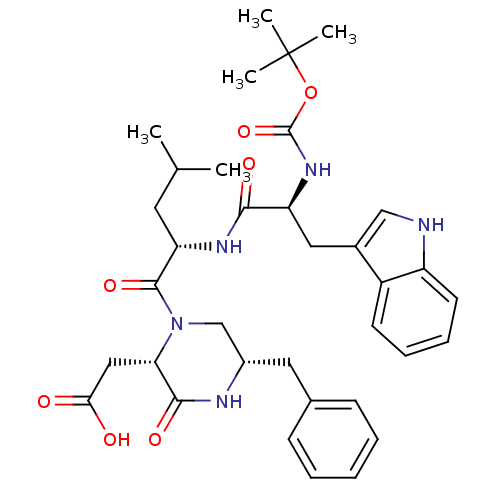
(((2S,5S)-5-Benzyl-1-{(S)-2-[(S)-2-tert-butoxycarbo...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N1C[C@H](Cc2ccccc2)NC(=O)[C@@H]1CC(O)=O Show InChI InChI=1S/C35H45N5O7/c1-21(2)15-28(33(45)40-20-24(16-22-11-7-6-8-12-22)37-32(44)29(40)18-30(41)42)38-31(43)27(39-34(46)47-35(3,4)5)17-23-19-36-26-14-10-9-13-25(23)26/h6-14,19,21,24,27-29,36H,15-18,20H2,1-5H3,(H,37,44)(H,38,43)(H,39,46)(H,41,42)/t24-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287246
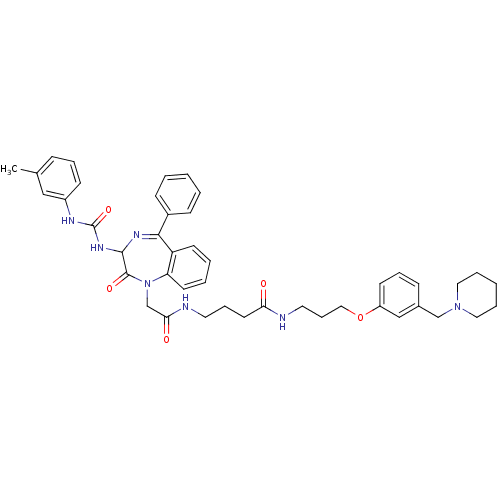
(4-{2-[2-Oxo-5-phenyl-3-(3-m-tolyl-ureido)-2,3-dihy...)Show SMILES Cc1cccc(NC(=O)NC2N=C(c3ccccc3)c3ccccc3N(CC(=O)NCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)C2=O)c1 |t:11| Show InChI InChI=1S/C44H51N7O5/c1-32-14-10-18-35(28-32)47-44(55)49-42-43(54)51(38-21-7-6-20-37(38)41(48-42)34-16-4-2-5-17-34)31-40(53)46-23-12-22-39(52)45-24-13-27-56-36-19-11-15-33(29-36)30-50-25-8-3-9-26-50/h2,4-7,10-11,14-21,28-29,42H,3,8-9,12-13,22-27,30-31H2,1H3,(H,45,52)(H,46,53)(H2,47,49,55) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287271

((3-{2-[2-(2,2-Diamino-vinyl)-thiazol-4-ylmethylsul...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1cccc(OC(=O)NCCCC(=O)NCCSCc2csc(CC(N)=N)n2)c1 |c:9| Show InChI InChI=1S/C37H41N9O5S2/c1-23-8-5-10-25(18-23)43-36(49)45-34-35(48)46(2)29-13-4-3-12-28(29)33(44-34)24-9-6-11-27(19-24)51-37(50)41-15-7-14-31(47)40-16-17-52-21-26-22-53-32(42-26)20-30(38)39/h3-6,8-13,18-19,22,34H,7,14-17,20-21H2,1-2H3,(H3,38,39)(H,40,47)(H,41,50)(H2,43,45,49)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287239
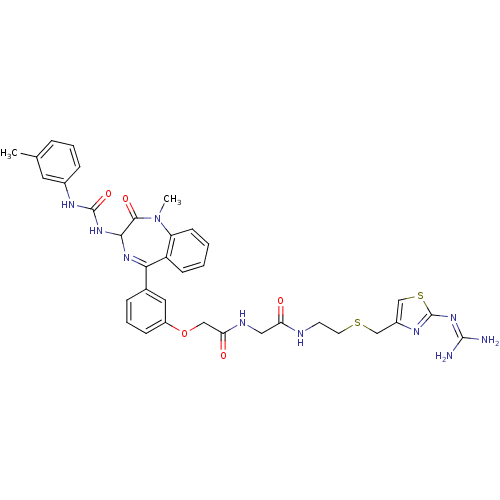
(CHEMBL30656 | N-{[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6])c2)-[#6]-1=O)-c1cccc(-[#8]-[#6]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c2csc(\[#7]=[#6](/[#7])-[#7])n2)c1 |c:9| Show InChI InChI=1S/C35H38N10O5S2/c1-21-7-5-9-23(15-21)40-34(49)43-31-32(48)45(2)27-12-4-3-11-26(27)30(42-31)22-8-6-10-25(16-22)50-18-29(47)39-17-28(46)38-13-14-51-19-24-20-52-35(41-24)44-33(36)37/h3-12,15-16,20,31H,13-14,17-19H2,1-2H3,(H,38,46)(H,39,47)(H2,40,43,49)(H4,36,37,41,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287252
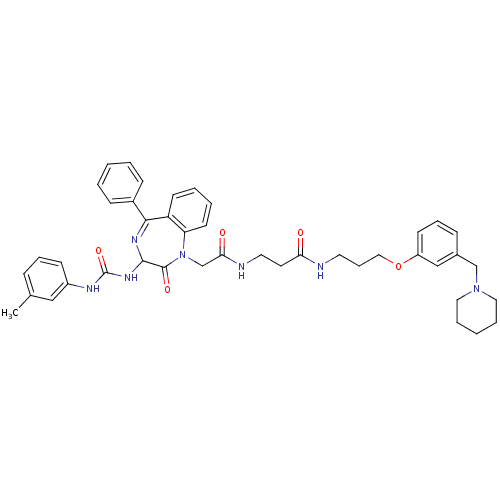
(3-{2-[2-Oxo-5-phenyl-3-(3-m-tolyl-ureido)-2,3-dihy...)Show SMILES Cc1cccc(NC(=O)NC2N=C(c3ccccc3)c3ccccc3N(CC(=O)NCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)C2=O)c1 |t:11| Show InChI InChI=1S/C43H49N7O5/c1-31-13-10-17-34(27-31)46-43(54)48-41-42(53)50(37-20-7-6-19-36(37)40(47-41)33-15-4-2-5-16-33)30-39(52)45-23-21-38(51)44-22-12-26-55-35-18-11-14-32(28-35)29-49-24-8-3-9-25-49/h2,4-7,10-11,13-20,27-28,41H,3,8-9,12,21-26,29-30H2,1H3,(H,44,51)(H,45,52)(H2,46,48,54) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287261
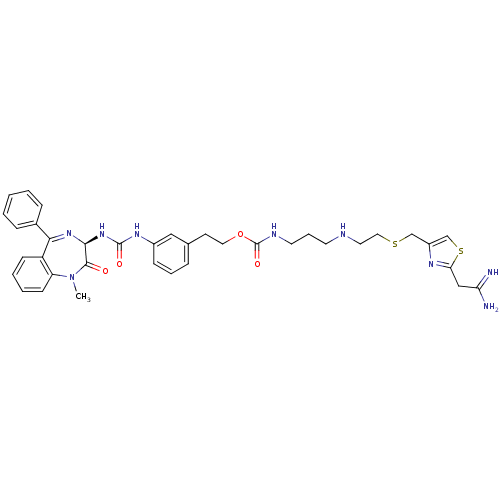
((3-{2-[2-(2,2-Diamino-vinyl)-thiazol-4-ylmethylsul...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CCOC(=O)NCCCNCCSCc3csc(CC(N)=N)n3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C37H43N9O4S2/c1-46-30-14-6-5-13-29(30)33(26-10-3-2-4-11-26)44-34(35(46)47)45-36(48)43-27-12-7-9-25(21-27)15-19-50-37(49)41-17-8-16-40-18-20-51-23-28-24-52-32(42-28)22-31(38)39/h2-7,9-14,21,24,34,40H,8,15-20,22-23H2,1H3,(H3,38,39)(H,41,49)(H2,43,45,48)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284159
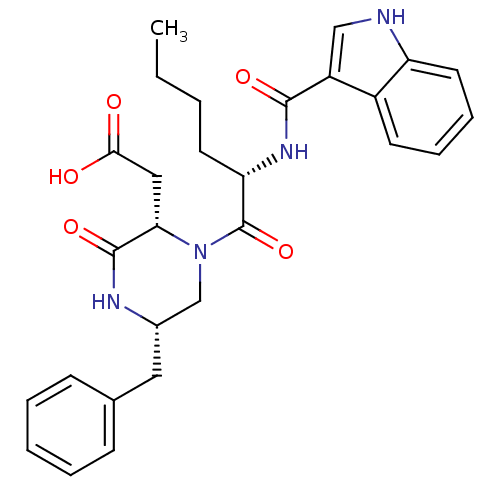
(((2S,5S)-5-Benzyl-1-{(S)-2-[(1H-indole-3-carbonyl)...)Show SMILES CCCC[C@H](NC(=O)c1c[nH]c2ccccc12)C(=O)N1C[C@H](Cc2ccccc2)NC(=O)[C@@H]1CC(O)=O Show InChI InChI=1S/C28H32N4O5/c1-2-3-12-23(31-26(35)21-16-29-22-13-8-7-11-20(21)22)28(37)32-17-19(14-18-9-5-4-6-10-18)30-27(36)24(32)15-25(33)34/h4-11,13,16,19,23-24,29H,2-3,12,14-15,17H2,1H3,(H,30,36)(H,31,35)(H,33,34)/t19-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287273
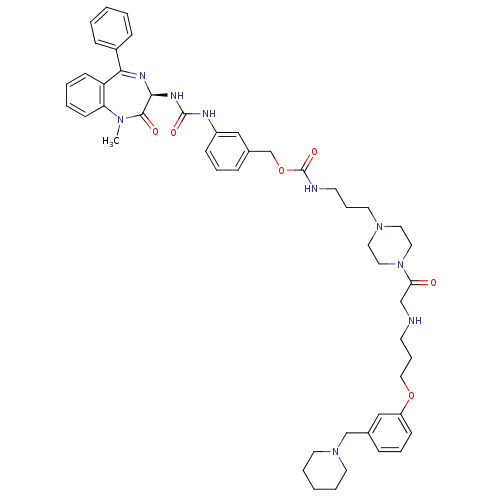
(CHEMBL285719 | [3-(4-{2-[3-(3-Piperidin-1-ylmethyl...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCCN3CCN(CC3)C(=O)CNCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C49H61N9O6/c1-55-43-21-7-6-20-42(43)45(39-16-4-2-5-17-39)53-46(47(55)60)54-48(61)52-40-18-10-15-38(32-40)36-64-49(62)51-23-12-26-56-27-29-58(30-28-56)44(59)34-50-22-13-31-63-41-19-11-14-37(33-41)35-57-24-8-3-9-25-57/h2,4-7,10-11,14-21,32-33,46,50H,3,8-9,12-13,22-31,34-36H2,1H3,(H,51,62)(H2,52,54,61)/t46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287245

(CHEMBL285586 | N-[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6])c2)-[#6]-1=O)-c1cccc(-[#8]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c2csc(\[#7]=[#6](/[#7])-[#7])n2)c1 |c:9| Show InChI InChI=1S/C36H40N10O5S2/c1-22-7-5-9-24(17-22)41-35(50)44-32-33(49)46(2)28-12-4-3-11-27(28)31(43-32)23-8-6-10-26(18-23)51-19-30(48)39-14-13-29(47)40-15-16-52-20-25-21-53-36(42-25)45-34(37)38/h3-12,17-18,21,32H,13-16,19-20H2,1-2H3,(H,39,48)(H,40,47)(H2,41,44,50)(H4,37,38,42,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287232
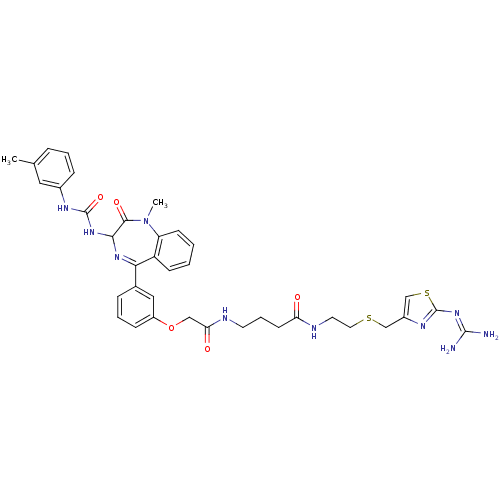
(CHEMBL31726 | N-[2-(2-Guanidino-thiazol-4-ylmethyl...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6])c2)-[#6]-1=O)-c1cccc(-[#8]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c2csc(\[#7]=[#6](\[#7])-[#7])n2)c1 |c:9| Show InChI InChI=1S/C37H42N10O5S2/c1-23-8-5-10-25(18-23)42-36(51)45-33-34(50)47(2)29-13-4-3-12-28(29)32(44-33)24-9-6-11-27(19-24)52-20-31(49)40-15-7-14-30(48)41-16-17-53-21-26-22-54-37(43-26)46-35(38)39/h3-6,8-13,18-19,22,33H,7,14-17,20-21H2,1-2H3,(H,40,49)(H,41,48)(H2,42,45,51)(H4,38,39,43,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287258

(3-[3-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-b...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(c2)C(=O)NCCCOc2cccc(CN3CCCCC3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C39H42N6O4/c1-44-34-20-7-6-19-33(34)35(29-14-4-2-5-15-29)42-36(38(44)47)43-39(48)41-31-17-11-16-30(26-31)37(46)40-21-12-24-49-32-18-10-13-28(25-32)27-45-22-8-3-9-23-45/h2,4-7,10-11,13-20,25-26,36H,3,8-9,12,21-24,27H2,1H3,(H,40,46)(H2,41,43,48)/t36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284150

(((2R,5S)-5-Benzyl-1-{(S)-2-[(S)-2-tert-butoxycarbo...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N1C[C@H](Cc2ccccc2)NC(=O)[C@H]1CC(O)=O Show InChI InChI=1S/C35H45N5O7/c1-21(2)15-28(33(45)40-20-24(16-22-11-7-6-8-12-22)37-32(44)29(40)18-30(41)42)38-31(43)27(39-34(46)47-35(3,4)5)17-23-19-36-26-14-10-9-13-25(23)26/h6-14,19,21,24,27-29,36H,15-18,20H2,1-5H3,(H,37,44)(H,38,43)(H,39,46)(H,41,42)/t24-,27-,28-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287270

(CHEMBL432671 | [(2-{2-[2-(2,2-Diamino-vinyl)-thiaz...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCNC(=O)CC(=O)NCCSCc3csc(CC(N)=N)n3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C37H40N10O6S2/c1-47-28-13-6-5-12-27(28)33(24-9-3-2-4-10-24)45-34(35(47)50)46-36(51)44-25-11-7-8-23(16-25)19-53-37(52)42-22-41-31(49)18-30(48)40-14-15-54-20-26-21-55-32(43-26)17-29(38)39/h2-13,16,21,34H,14-15,17-20,22H2,1H3,(H3,38,39)(H,40,48)(H,41,49)(H,42,52)(H2,44,46,51)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50019202
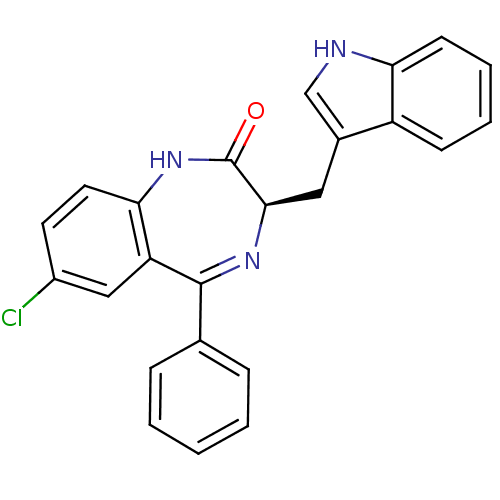
(7-Chloro-3-(1H-indol-3-ylmethyl)-5-phenyl-1,3-dihy...)Show SMILES Clc1ccc2NC(=O)[C@@H](Cc3c[nH]c4ccccc34)N=C(c3ccccc3)c2c1 |t:21| Show InChI InChI=1S/C24H18ClN3O/c25-17-10-11-21-19(13-17)23(15-6-2-1-3-7-15)27-22(24(29)28-21)12-16-14-26-20-9-5-4-8-18(16)20/h1-11,13-14,22,26H,12H2,(H,28,29)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Half-maximal inhibition of [125I]CCK-33 binding to cholecystokinin receptor |
J Med Chem 31: 2235-46 (1989)
BindingDB Entry DOI: 10.7270/Q2PG1S9K |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50280054
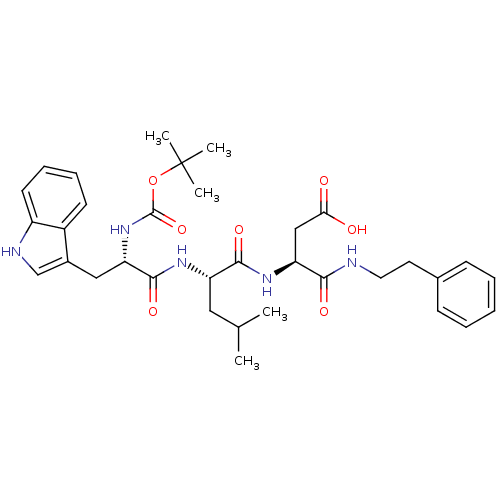
((S)-3-((S)-2-((S)-2-(tert-butoxycarbonyl)-3-(1H-in...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C34H45N5O7/c1-21(2)17-26(31(43)38-28(19-29(40)41)30(42)35-16-15-22-11-7-6-8-12-22)37-32(44)27(39-33(45)46-34(3,4)5)18-23-20-36-25-14-10-9-13-24(23)25/h6-14,20-21,26-28,36H,15-19H2,1-5H3,(H,35,42)(H,37,44)(H,38,43)(H,39,45)(H,40,41)/t26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50280054

((S)-3-((S)-2-((S)-2-(tert-butoxycarbonyl)-3-(1H-in...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C34H45N5O7/c1-21(2)17-26(31(43)38-28(19-29(40)41)30(42)35-16-15-22-11-7-6-8-12-22)37-32(44)27(39-33(45)46-34(3,4)5)18-23-20-36-25-14-10-9-13-24(23)25/h6-14,20-21,26-28,36H,15-19H2,1-5H3,(H,35,42)(H,37,44)(H,38,43)(H,39,45)(H,40,41)/t26-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration of compound required to inhibit binding of [125I]J-BH-CCK-8 radioligand to CCKA in mouse pancreatic membranes |
Bioorg Med Chem Lett 2: 9-12 (1992)
Article DOI: 10.1016/S0960-894X(00)80644-7
BindingDB Entry DOI: 10.7270/Q29P3242 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50284155

(((2S,5R)-5-Benzyl-1-{(S)-2-[(S)-2-tert-butoxycarbo...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N1C[C@@H](Cc2ccccc2)NC(=O)[C@@H]1CC(O)=O Show InChI InChI=1S/C35H45N5O7/c1-21(2)15-28(33(45)40-20-24(16-22-11-7-6-8-12-22)37-32(44)29(40)18-30(41)42)38-31(43)27(39-34(46)47-35(3,4)5)17-23-19-36-26-14-10-9-13-25(23)26/h6-14,19,21,24,27-29,36H,15-18,20H2,1-5H3,(H,37,44)(H,38,43)(H,39,46)(H,41,42)/t24-,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Concentration producing half-maximal inhibition of specific binding of [125I]- CCK-8 to CCK receptors on mouse pancreatic membranes (CCK-A) |
Bioorg Med Chem Lett 4: 867-872 (1994)
Article DOI: 10.1016/S0960-894X(01)80253-5
BindingDB Entry DOI: 10.7270/Q2WQ03QK |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287269
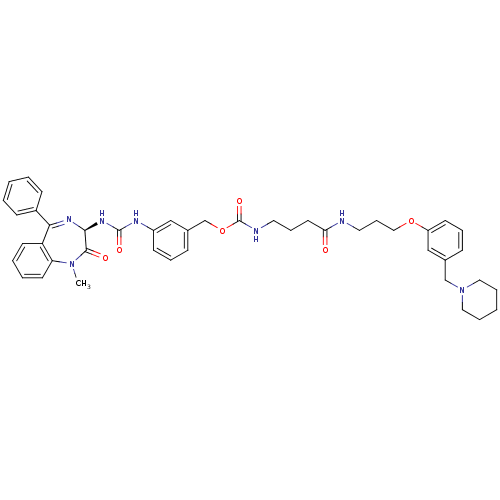
(CHEMBL33698 | {3-[3-(3-Piperidin-1-ylmethyl-phenox...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(COC(=O)NCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C44H51N7O6/c1-50-38-21-7-6-20-37(38)40(34-16-4-2-5-17-34)48-41(42(50)53)49-43(54)47-35-18-10-15-33(28-35)31-57-44(55)46-23-12-22-39(52)45-24-13-27-56-36-19-11-14-32(29-36)30-51-25-8-3-9-26-51/h2,4-7,10-11,14-21,28-29,41H,3,8-9,12-13,22-27,30-31H2,1H3,(H,45,52)(H,46,55)(H2,47,49,54)/t41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287240
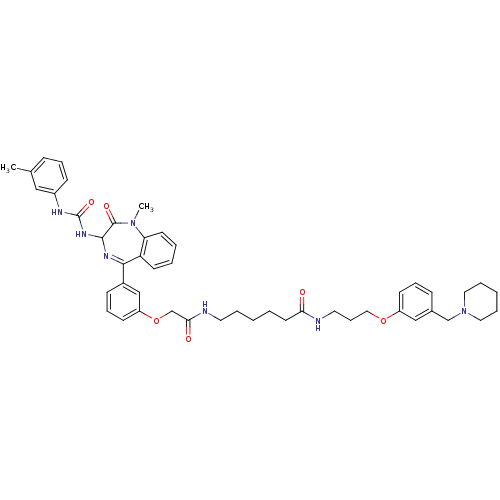
(6-(2-{3-[1-Methyl-2-oxo-3-(3-m-tolyl-ureido)-2,3-d...)Show SMILES CN1c2ccccc2C(=NC(NC(=O)Nc2cccc(C)c2)C1=O)c1cccc(OCC(=O)NCCCCCC(=O)NCCCOc2cccc(CN3CCCCC3)c2)c1 |c:9| Show InChI InChI=1S/C47H57N7O6/c1-34-15-11-18-37(29-34)50-47(58)52-45-46(57)53(2)41-22-7-6-21-40(41)44(51-45)36-17-13-20-39(31-36)60-33-43(56)49-24-8-3-5-23-42(55)48-25-14-28-59-38-19-12-16-35(30-38)32-54-26-9-4-10-27-54/h6-7,11-13,15-22,29-31,45H,3-5,8-10,14,23-28,32-33H2,1-2H3,(H,48,55)(H,49,56)(H2,50,52,58) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287264

(CHEMBL287295 | {3-[3-(3-Piperidin-1-ylmethyl-pheno...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1cccc(OC(=O)NCCCC(=O)NCCCOc2cccc(CN3CCCCC3)c2)c1 |c:9| Show InChI InChI=1S/C44H51N7O6/c1-31-13-8-16-34(27-31)47-43(54)49-41-42(53)50(2)38-20-5-4-19-37(38)40(48-41)33-15-10-18-36(29-33)57-44(55)46-22-11-21-39(52)45-23-12-26-56-35-17-9-14-32(28-35)30-51-24-6-3-7-25-51/h4-5,8-10,13-20,27-29,41H,3,6-7,11-12,21-26,30H2,1-2H3,(H,45,52)(H,46,55)(H2,47,49,54)/t41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287257
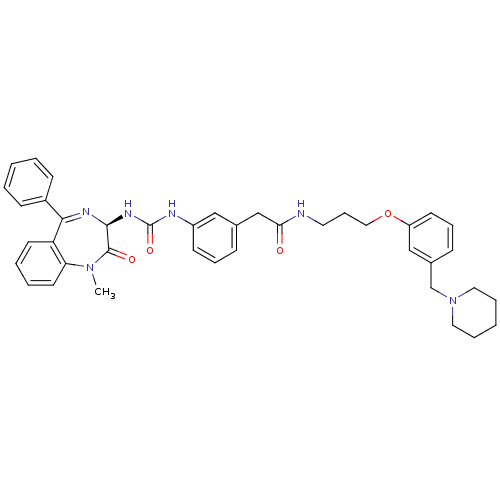
(2-{3-[3-((R)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C40H44N6O4/c1-45-35-20-7-6-19-34(35)37(31-15-4-2-5-16-31)43-38(39(45)48)44-40(49)42-32-17-10-13-29(25-32)27-36(47)41-21-12-24-50-33-18-11-14-30(26-33)28-46-22-8-3-9-23-46/h2,4-7,10-11,13-20,25-26,38H,3,8-9,12,21-24,27-28H2,1H3,(H,41,47)(H2,42,44,49)/t38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287267

(CHEMBL284975 | {2-[3-(3-Piperidin-1-ylmethyl-pheno...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CCOC(=O)NCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C44H51N7O6/c1-50-38-20-7-6-19-37(38)40(34-15-4-2-5-16-34)48-41(42(50)53)49-43(54)47-35-17-10-13-32(29-35)22-28-57-44(55)46-24-21-39(52)45-23-12-27-56-36-18-11-14-33(30-36)31-51-25-8-3-9-26-51/h2,4-7,10-11,13-20,29-30,41H,3,8-9,12,21-28,31H2,1H3,(H,45,52)(H,46,55)(H2,47,49,54)/t41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287260
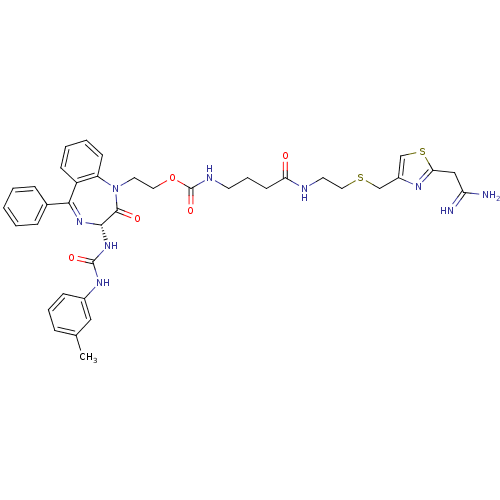
((3-{2-[2-(2,2-Diamino-vinyl)-thiazol-4-ylmethylsul...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CCOC(=O)NCCCC(=O)NCCSCc3csc(CC(N)=N)n3)C2=O)c1 |t:11| Show InChI InChI=1S/C38H43N9O5S2/c1-25-9-7-12-27(21-25)44-37(50)46-35-36(49)47(30-14-6-5-13-29(30)34(45-35)26-10-3-2-4-11-26)18-19-52-38(51)42-16-8-15-32(48)41-17-20-53-23-28-24-54-33(43-28)22-31(39)40/h2-7,9-14,21,24,35H,8,15-20,22-23H2,1H3,(H3,39,40)(H,41,48)(H,42,51)(H2,44,46,50)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287262

(CHEMBL33743 | {3-[3-(3-Piperidin-1-ylmethyl-phenox...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CCOC(=O)NCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)C2=O)c1 |t:11| Show InChI InChI=1S/C45H53N7O6/c1-33-14-10-18-36(30-33)48-44(55)50-42-43(54)52(39-21-7-6-20-38(39)41(49-42)35-16-4-2-5-17-35)27-29-58-45(56)47-23-12-22-40(53)46-24-13-28-57-37-19-11-15-34(31-37)32-51-25-8-3-9-26-51/h2,4-7,10-11,14-21,30-31,42H,3,8-9,12-13,22-29,32H2,1H3,(H,46,53)(H,47,56)(H2,48,50,55)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287241
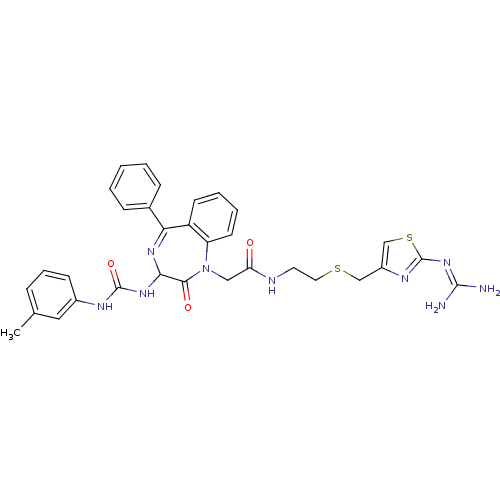
(CHEMBL285746 | N-[2-(2-Guanidino-thiazol-4-ylmethy...)Show SMILES [#6]-c1cccc(-[#7]-[#6](=O)-[#7]-[#6]-2-[#7]=[#6](-c3ccccc3)-c3ccccc3-[#7](-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c3csc(\[#7]=[#6](\[#7])-[#7])n3)-[#6]-2=O)c1 |t:11| Show InChI InChI=1S/C32H33N9O3S2/c1-20-8-7-11-22(16-20)36-31(44)39-28-29(43)41(25-13-6-5-12-24(25)27(38-28)21-9-3-2-4-10-21)17-26(42)35-14-15-45-18-23-19-46-32(37-23)40-30(33)34/h2-13,16,19,28H,14-15,17-18H2,1H3,(H,35,42)(H2,36,39,44)(H4,33,34,37,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287266
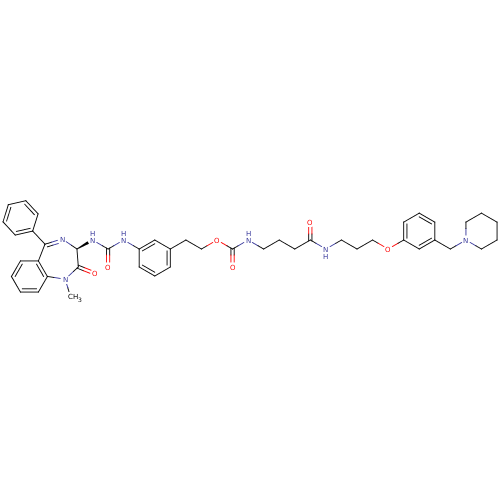
(CHEMBL290122 | {3-[3-(3-Piperidin-1-ylmethyl-pheno...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(CCOC(=O)NCCCC(=O)NCCCOc3cccc(CN4CCCCC4)c3)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C45H53N7O6/c1-51-39-21-7-6-20-38(39)41(35-16-4-2-5-17-35)49-42(43(51)54)50-44(55)48-36-18-10-14-33(30-36)23-29-58-45(56)47-24-12-22-40(53)46-25-13-28-57-37-19-11-15-34(31-37)32-52-26-8-3-9-27-52/h2,4-7,10-11,14-21,30-31,42H,3,8-9,12-13,22-29,32H2,1H3,(H,46,53)(H,47,56)(H2,48,50,55)/t42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1427-1430 (1996)
Article DOI: 10.1016/S0960-894X(96)00249-1
BindingDB Entry DOI: 10.7270/Q2XD11NF |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Mus musculus) | BDBM50287243
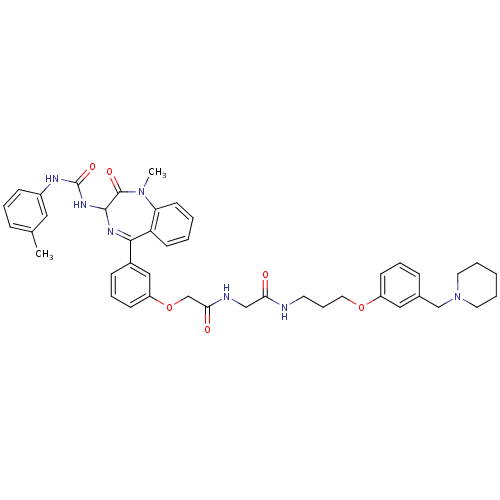
(2-{3-[1-Methyl-2-oxo-3-(3-m-tolyl-ureido)-2,3-dihy...)Show SMILES CN1c2ccccc2C(=NC(NC(=O)Nc2cccc(C)c2)C1=O)c1cccc(OCC(=O)NCC(=O)NCCCOc2cccc(CN3CCCCC3)c2)c1 |c:9| Show InChI InChI=1S/C43H49N7O6/c1-30-12-8-15-33(24-30)46-43(54)48-41-42(53)49(2)37-19-5-4-18-36(37)40(47-41)32-14-10-17-35(26-32)56-29-39(52)45-27-38(51)44-20-11-23-55-34-16-9-13-31(25-34)28-50-21-6-3-7-22-50/h4-5,8-10,12-19,24-26,41H,3,6-7,11,20-23,27-29H2,1-2H3,(H,44,51)(H,45,52)(H2,46,48,54) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
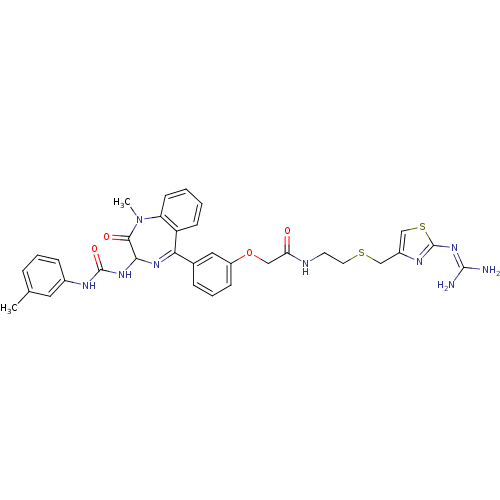
(Mus musculus) | BDBM50287237
(CHEMBL29888 | N-[2-(2-Guanidino-thiazol-4-ylmethyl...)Show SMILES [#6]-[#7]-1-c2ccccc2-[#6](=[#7]-[#6](-[#7]-[#6](=O)-[#7]-c2cccc(-[#6])c2)-[#6]-1=O)-c1cccc(-[#8]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-c2csc(\[#7]=[#6](/[#7])-[#7])n2)c1 |c:9| Show InChI InChI=1S/C33H35N9O4S2/c1-20-7-5-9-22(15-20)37-32(45)40-29-30(44)42(2)26-12-4-3-11-25(26)28(39-29)21-8-6-10-24(16-21)46-17-27(43)36-13-14-47-18-23-19-48-33(38-23)41-31(34)35/h3-12,15-16,19,29H,13-14,17-18H2,1-2H3,(H,36,43)(H2,37,40,45)(H4,34,35,38,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cholecystokinin type A receptor |
Bioorg Med Chem Lett 6: 1421-1426 (1996)
Article DOI: 10.1016/S0960-894X(96)00248-X
BindingDB Entry DOI: 10.7270/Q2251J56 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data