

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50061067 (15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay | Bioorg Med Chem Lett 13: 2903-6 (2003) BindingDB Entry DOI: 10.7270/Q2X63MB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50090297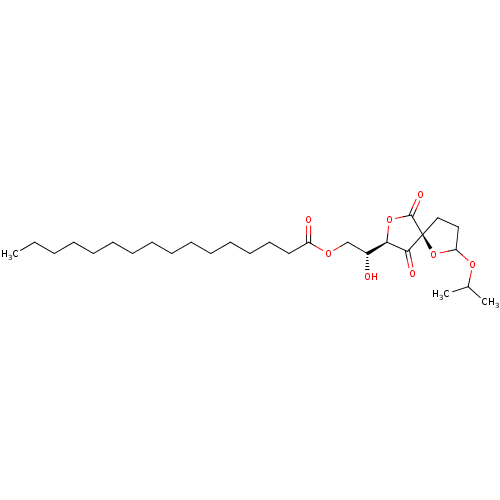 (CHEMBL40779 | Hexadecanoic acid (R)-2-hydroxy-2-((...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Göttingen Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against protein phosphatase I (PP-I) | Bioorg Med Chem Lett 10: 1605-8 (2000) BindingDB Entry DOI: 10.7270/Q2CN7348 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50090298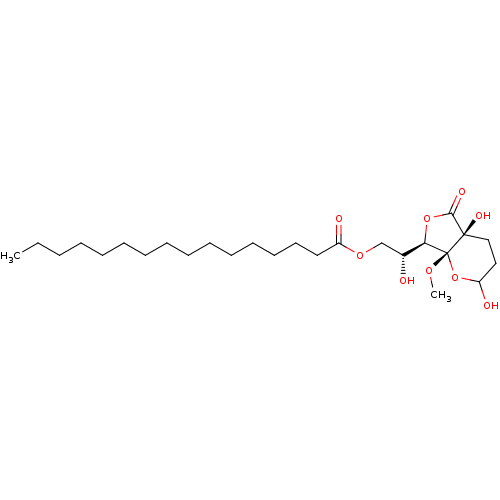 (CHEMBL416976 | Hexadecanoic acid (R)-2-((4aS,7R,7a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Göttingen Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against protein phosphatase I (PP-I) | Bioorg Med Chem Lett 10: 1605-8 (2000) BindingDB Entry DOI: 10.7270/Q2CN7348 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50183456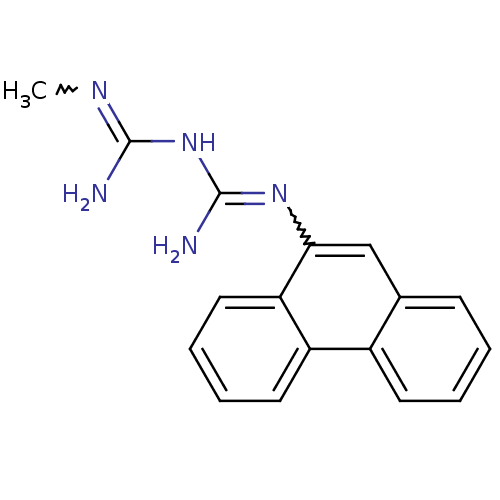 (CHEMBL425403 | N-methyl-N'-9-phenanthrylimidodicar...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Rockefeller University Curated by ChEMBL | Assay Description Inhibition of PP2C alpha | J Med Chem 49: 1658-67 (2006) Article DOI: 10.1021/jm051033y BindingDB Entry DOI: 10.7270/Q20001QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50343530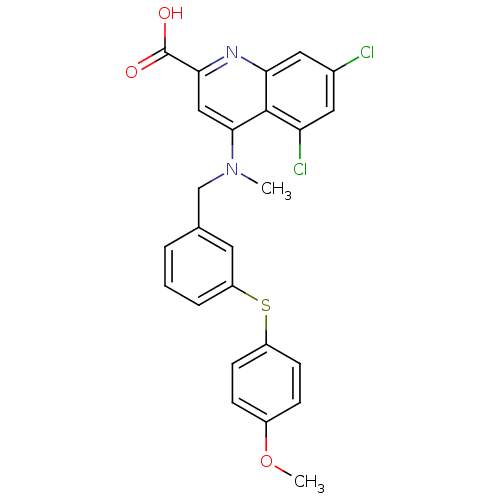 (5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of PPM1A (unknown origin) assessed as reduction in pNPP hydrolysis | J Med Chem 57: 9309-22 (2014) Article DOI: 10.1021/jm500692u BindingDB Entry DOI: 10.7270/Q2ZG6TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50031908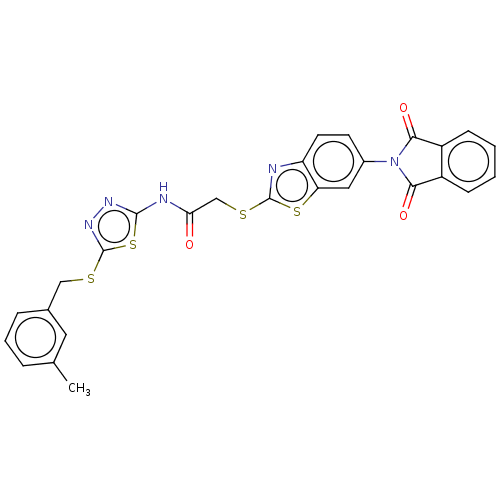 (CHEMBL3360906) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of PPM1A (unknown origin) assessed as reduction in pNPP hydrolysis | J Med Chem 57: 9309-22 (2014) Article DOI: 10.1021/jm500692u BindingDB Entry DOI: 10.7270/Q2ZG6TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50031910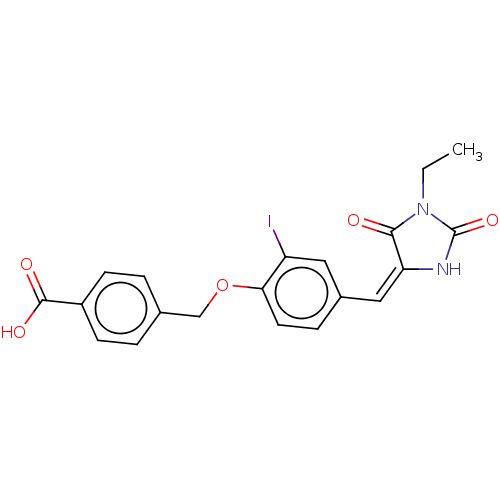 (CHEMBL3360908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of PPM1A (unknown origin) assessed as reduction in pNPP hydrolysis | J Med Chem 57: 9309-22 (2014) Article DOI: 10.1021/jm500692u BindingDB Entry DOI: 10.7270/Q2ZG6TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50135681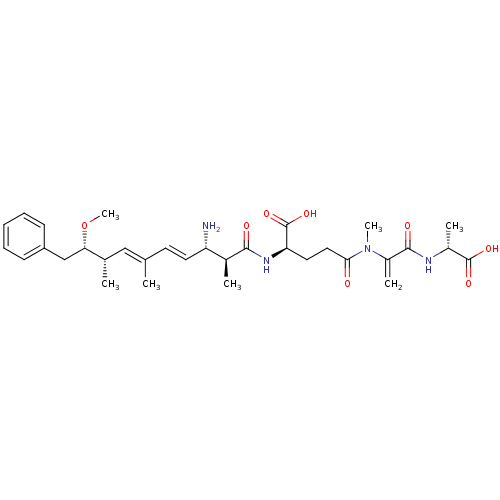 ((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Amino-9-methoxy-2,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay | Bioorg Med Chem Lett 13: 2903-6 (2003) BindingDB Entry DOI: 10.7270/Q2X63MB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50031906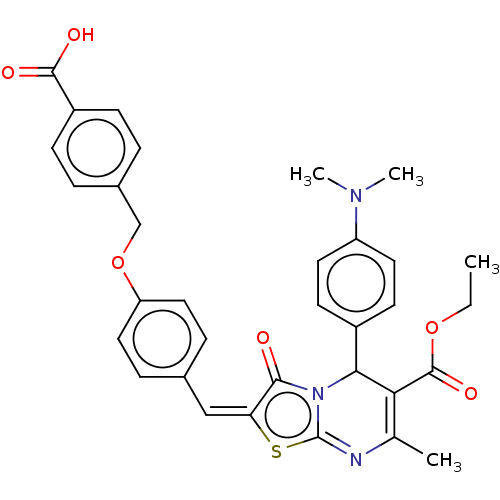 (CHEMBL3360905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of PPM1A (unknown origin) assessed as reduction in pNPP hydrolysis | J Med Chem 57: 9309-22 (2014) Article DOI: 10.1021/jm500692u BindingDB Entry DOI: 10.7270/Q2ZG6TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50135676 ((S)-2-[(R)-2-(2-{[(R)-4-((4E,6E)-(2S,3S,8S,9S)-3-A...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay | Bioorg Med Chem Lett 13: 2903-6 (2003) BindingDB Entry DOI: 10.7270/Q2X63MB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50135680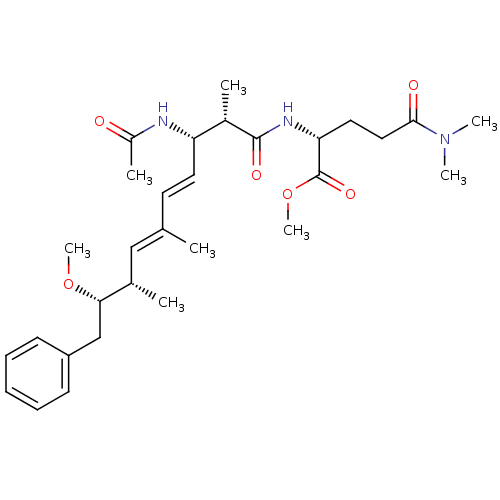 ((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay | Bioorg Med Chem Lett 13: 2903-6 (2003) BindingDB Entry DOI: 10.7270/Q2X63MB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50135677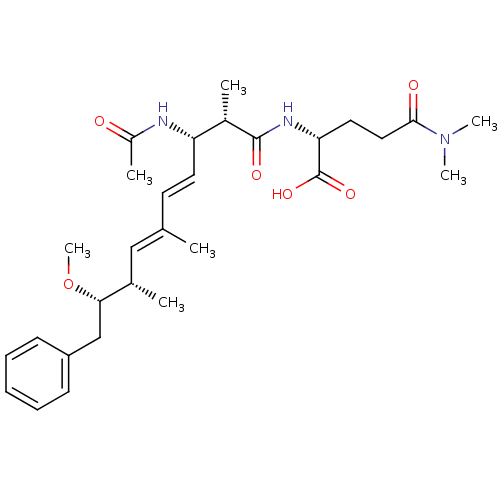 ((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay | Bioorg Med Chem Lett 13: 2903-6 (2003) BindingDB Entry DOI: 10.7270/Q2X63MB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50135675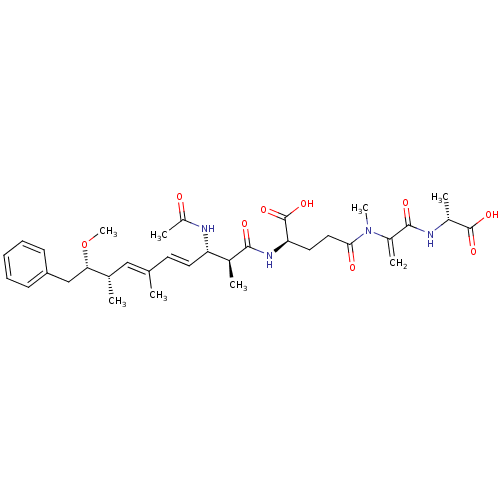 ((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay | Bioorg Med Chem Lett 13: 2903-6 (2003) BindingDB Entry DOI: 10.7270/Q2X63MB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50135679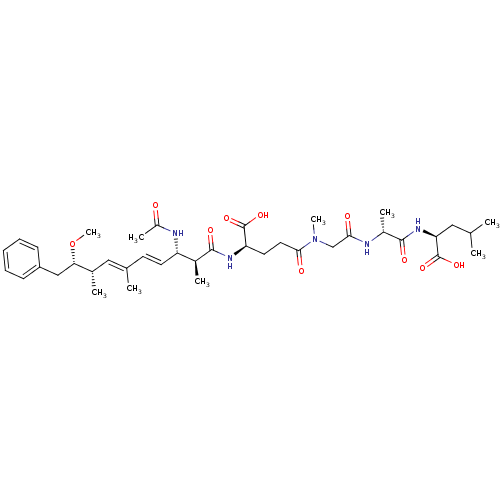 ((S)-2-[(R)-2-(2-{[(R)-4-((4E,6E)-(2S,3S,8S,9S)-3-A...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay | Bioorg Med Chem Lett 13: 2903-6 (2003) BindingDB Entry DOI: 10.7270/Q2X63MB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50135678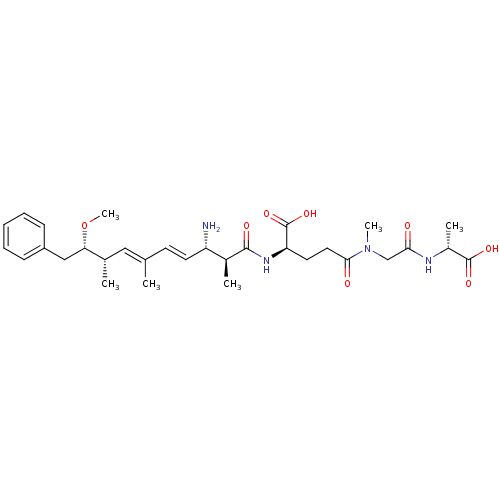 ((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Amino-9-methoxy-2,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay | Bioorg Med Chem Lett 13: 2903-6 (2003) BindingDB Entry DOI: 10.7270/Q2X63MB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein phosphatase 1A (Homo sapiens (Human)) | BDBM50135674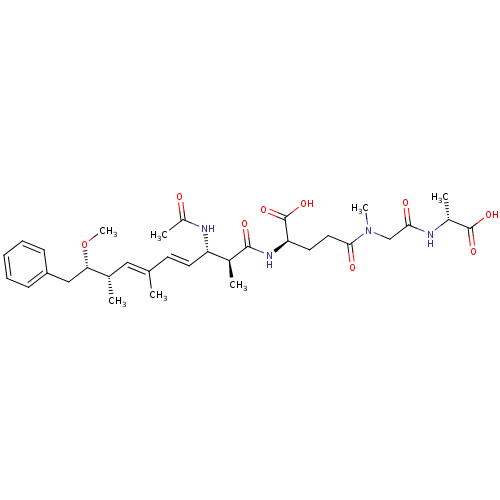 ((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine Curated by ChEMBL | Assay Description Inhibitory concentration of the compound required against protein phosphatase 1 using pNPP assay | Bioorg Med Chem Lett 13: 2903-6 (2003) BindingDB Entry DOI: 10.7270/Q2X63MB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||