

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225361 (Etisulergine) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against,[3H]spiperone rat frontal cortex (SPFC) serotonin receptor | J Med Chem 28: 1540-2 (1985) BindingDB Entry DOI: 10.7270/Q27M0B5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225361 (Etisulergine) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against serotonin receptor from rat frontal cortex using [3H]spiperone as radioligand | J Med Chem 28: 367-75 (1985) BindingDB Entry DOI: 10.7270/Q22V2J98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073906 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073894 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073893 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073885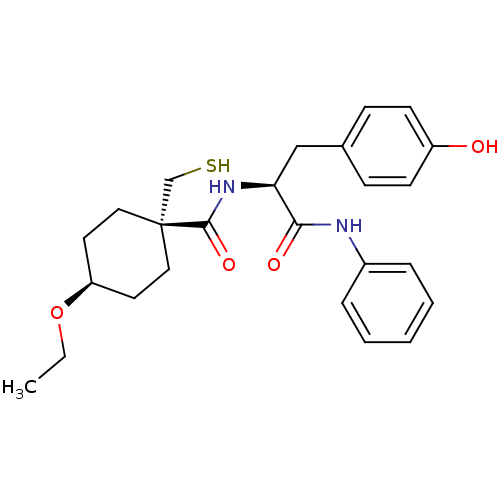 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073881 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073886 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073883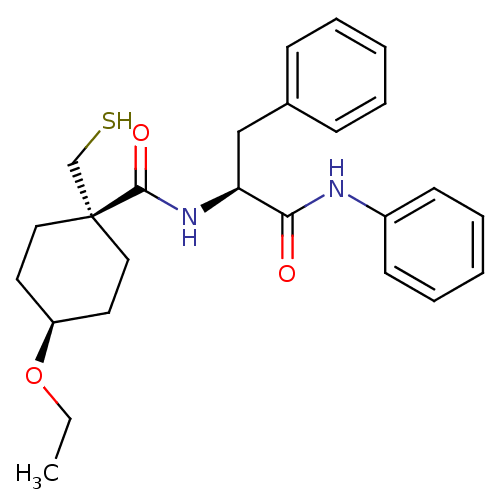 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073900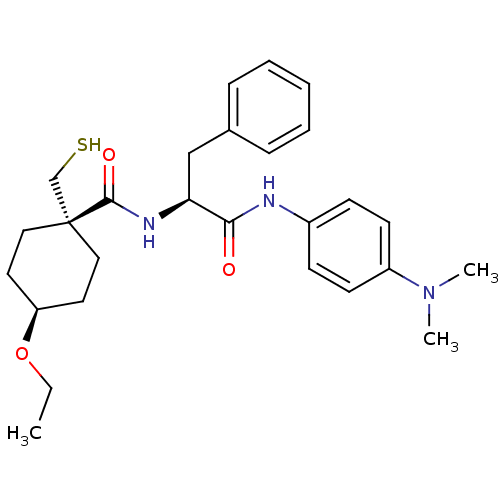 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073905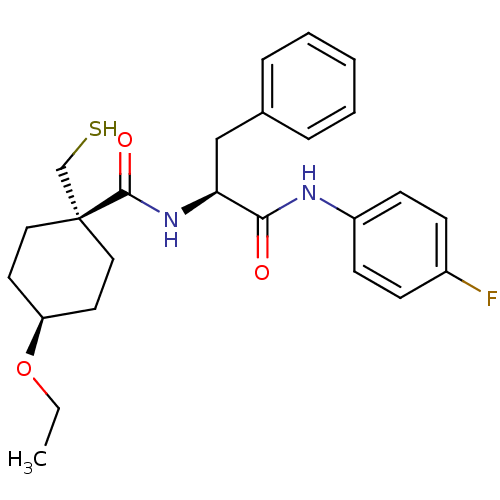 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073889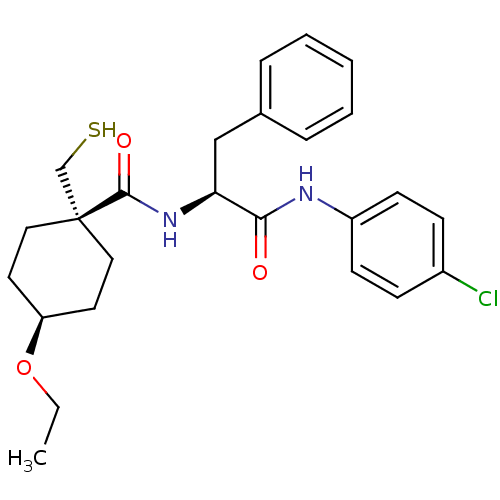 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073891 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073888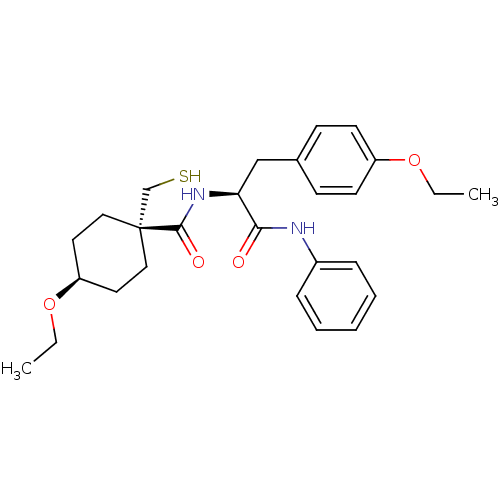 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50017543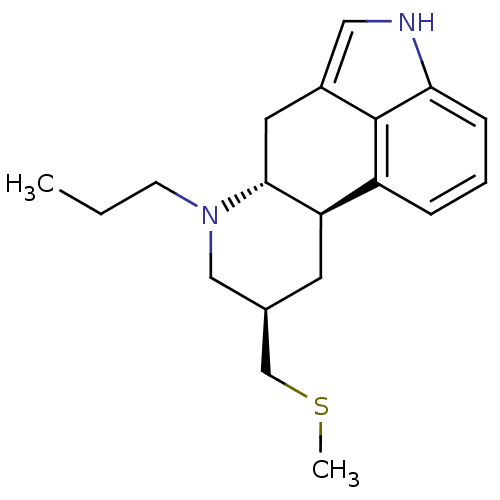 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of rat liver | J Med Chem 28: 367-75 (1985) BindingDB Entry DOI: 10.7270/Q22V2J98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073899 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073898 (1-Mercaptomethyl-4-propoxy-cyclohexanecarboxylic a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073895 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073887 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50017543 ((6aR,9R,10aR)-9-Methylsulfanylmethyl-7-propyl-4,6,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 385 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against serotonin receptor from whole rat brain using [3H]5-HT as radioligand | J Med Chem 28: 367-75 (1985) BindingDB Entry DOI: 10.7270/Q22V2J98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50291447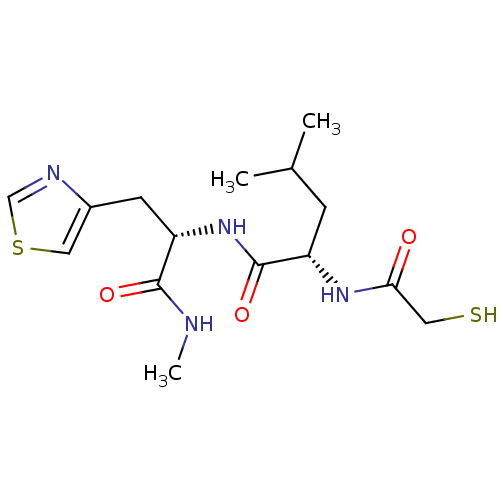 ((S)-2-(2-Mercapto-acetylamino)-4-methyl-pentanoic ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against Matrix metalloproteinase-3(MMP-3) in adjuvant arthritic rat model of rheumatoid arthritis | Bioorg Med Chem Lett 7: 897-902 (1997) Article DOI: 10.1016/S0960-894X(97)00125-X BindingDB Entry DOI: 10.7270/Q2PC32CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50291445 ((S)-2-(2-Mercapto-acetylamino)-4-methyl-pentanoic ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against Matrix metalloproteinase-3(MMP-3) in adjuvant arthritic rat model of rheumatoid arthritis | Bioorg Med Chem Lett 7: 897-902 (1997) Article DOI: 10.1016/S0960-894X(97)00125-X BindingDB Entry DOI: 10.7270/Q2PC32CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073902 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 449 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073904 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 568 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073882 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 617 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073892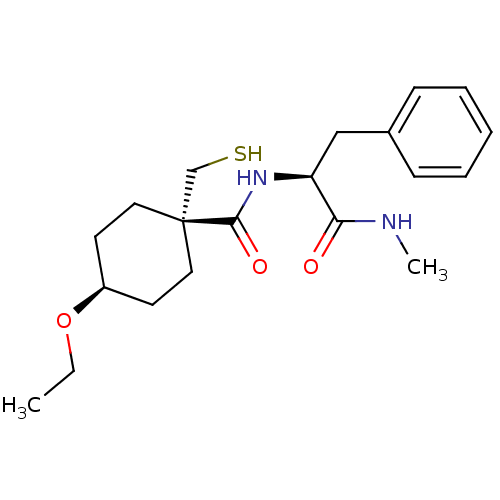 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50291446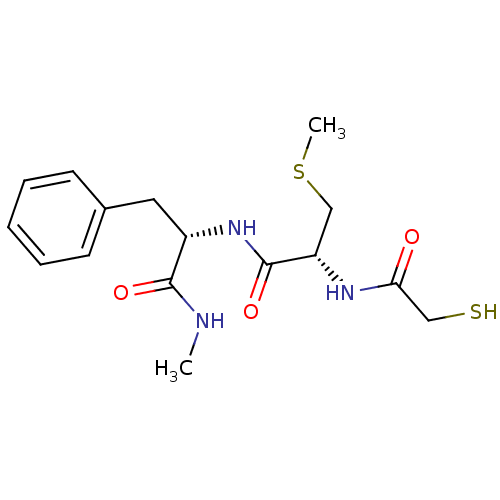 ((R)-2-(2-Mercapto-acetylamino)-N-((S)-1-methylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against Matrix metalloproteinase-3 (MMP-3) in adjuvant arthritic rat model of rheumatoid arthritis | Bioorg Med Chem Lett 7: 897-902 (1997) Article DOI: 10.1016/S0960-894X(97)00125-X BindingDB Entry DOI: 10.7270/Q2PC32CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073890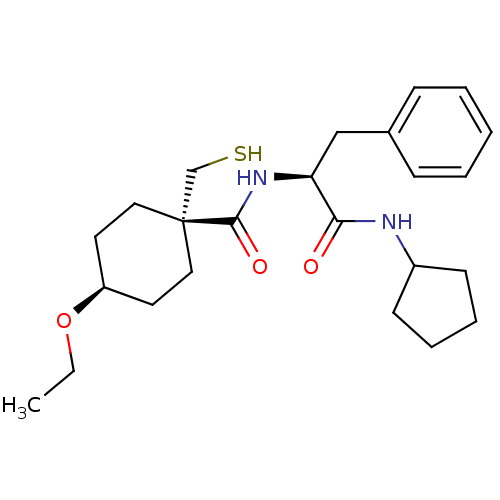 (4-Ethoxy-1-mercaptomethyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 666 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225476 (CHEMBL330849) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of rat liver | J Med Chem 28: 367-75 (1985) BindingDB Entry DOI: 10.7270/Q22V2J98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50291444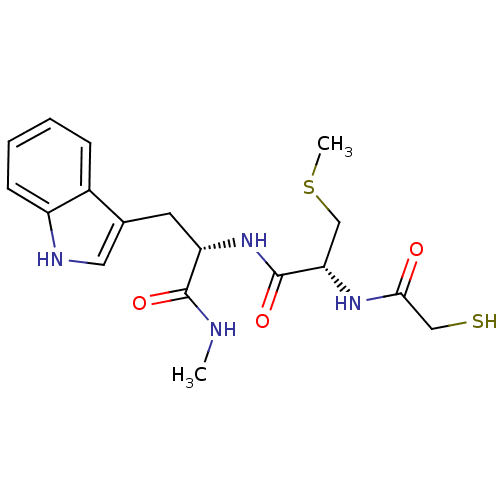 ((R)-N-[(S)-2-(1H-Indol-3-yl)-1-methylcarbamoyl-eth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against Matrix metalloproteinase-3(MMP-3) in adjuvant arthritic rat model of rheumatoid arthritis | Bioorg Med Chem Lett 7: 897-902 (1997) Article DOI: 10.1016/S0960-894X(97)00125-X BindingDB Entry DOI: 10.7270/Q2PC32CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073903 (1-Mercaptomethyl-4-methoxy-cyclohexanecarboxylic a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 926 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225476 (CHEMBL330849) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against serotonin receptor from whole rat brain using [3H]5-HT as radioligand | J Med Chem 28: 367-75 (1985) BindingDB Entry DOI: 10.7270/Q22V2J98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225362 (Norprolac | QUINAGOLIDE) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against,[3H]spiperone rat frontal cortex (SPFC) serotonin receptor | J Med Chem 28: 1540-2 (1985) BindingDB Entry DOI: 10.7270/Q27M0B5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225362 (Norprolac | QUINAGOLIDE) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against,[3H]spiperone rat frontal cortex (SPFC) serotonin receptor | J Med Chem 28: 1540-2 (1985) BindingDB Entry DOI: 10.7270/Q27M0B5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50290672 ((S)-2-(2-Mercapto-acetylamino)-4-methyl-pentanoic ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against Matrix metalloproteinase-3 (MMP-3) in adjuvant arthritic rat model of rheumatoid arthritis | Bioorg Med Chem Lett 7: 897-902 (1997) Article DOI: 10.1016/S0960-894X(97)00125-X BindingDB Entry DOI: 10.7270/Q2PC32CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225362 (Norprolac | QUINAGOLIDE) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against,[3H]serotonin (5-HT) whole rat brain serotonin receptor | J Med Chem 28: 1540-2 (1985) BindingDB Entry DOI: 10.7270/Q27M0B5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225362 (Norprolac | QUINAGOLIDE) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against,[3H]serotonin (5-HT) whole rat brain serotonin receptor | J Med Chem 28: 1540-2 (1985) BindingDB Entry DOI: 10.7270/Q27M0B5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073897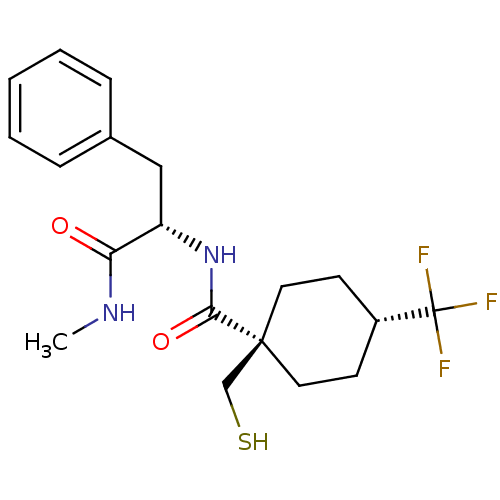 (1-Mercaptomethyl-4-trifluoromethyl-cyclohexanecarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against,[3H]spiperone rat frontal cortex (SPFC) serotonin receptor | J Med Chem 28: 1540-2 (1985) BindingDB Entry DOI: 10.7270/Q27M0B5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against serotonin receptor from rat frontal cortex using [3H]spiperone as radioligand | J Med Chem 28: 367-75 (1985) BindingDB Entry DOI: 10.7270/Q22V2J98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225362 (Norprolac | QUINAGOLIDE) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against serotonin receptor from whole rat brain using [3H]5-HT as radioligand | J Med Chem 28: 367-75 (1985) BindingDB Entry DOI: 10.7270/Q22V2J98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225361 (Etisulergine) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against,[3H]serotonin (5-HT) whole rat brain serotonin receptor | J Med Chem 28: 1540-2 (1985) BindingDB Entry DOI: 10.7270/Q27M0B5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225361 (Etisulergine) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of rat liver | J Med Chem 28: 367-75 (1985) BindingDB Entry DOI: 10.7270/Q22V2J98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225362 (Norprolac | QUINAGOLIDE) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against Dihydrofolate reductase of rat liver | J Med Chem 28: 367-75 (1985) BindingDB Entry DOI: 10.7270/Q22V2J98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50291443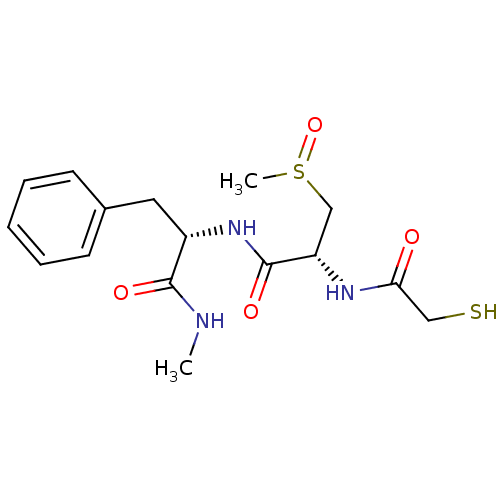 ((R)-2-(2-Mercapto-acetylamino)-3-methanesulfinyl-N...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against Matrix metalloproteinase-3 (MMP-3) in adjuvant arthritic rat model of rheumatoid arthritis | Bioorg Med Chem Lett 7: 897-902 (1997) Article DOI: 10.1016/S0960-894X(97)00125-X BindingDB Entry DOI: 10.7270/Q2PC32CH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073884 (1-Mercaptomethyl-cyclohexanecarboxylic acid ((S)-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50225362 (Norprolac | QUINAGOLIDE) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity was evaluated against,[3H]serotonin (5-HT) whole rat brain serotonin receptor | J Med Chem 28: 1540-2 (1985) BindingDB Entry DOI: 10.7270/Q27M0B5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Noncompetitive Inhibitory activity against rat striatal synaptosomal DHPR enzyme | J Med Chem 28: 367-75 (1985) BindingDB Entry DOI: 10.7270/Q22V2J98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073896 (1-Mercaptomethyl-4-propyl-cyclohexanecarboxylic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metalloproteinase inhibitor 3 (Rattus norvegicus) | BDBM50073901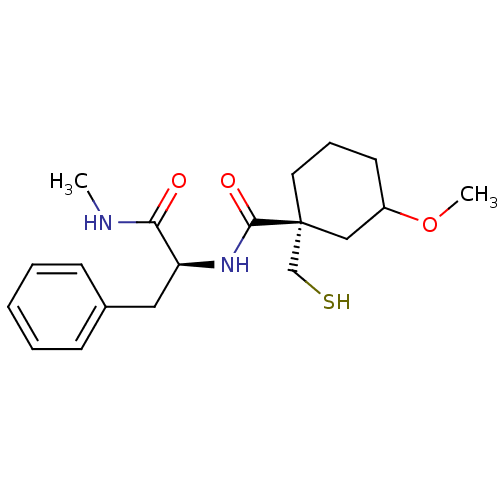 ((R)-1-Mercaptomethyl-3-methoxy-cyclohexanecarboxyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Biomedical Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against matrix metalloproteinase-3 | Bioorg Med Chem Lett 9: 195-200 (1999) BindingDB Entry DOI: 10.7270/Q2RX9B7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |