

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Rattus norvegicus) | BDBM50014777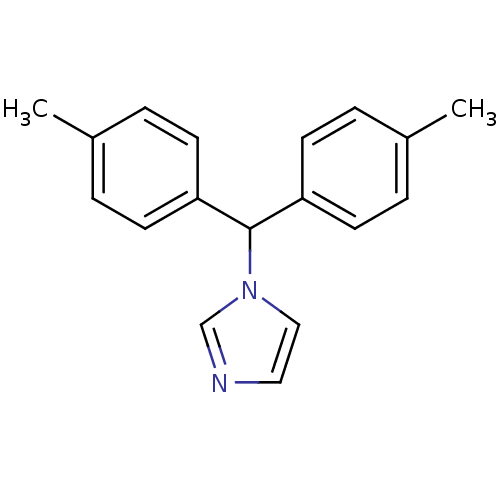 (1-Di-p-tolylmethyl-1H-imidazole | CHEMBL135381) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014754 (1-[(4-Chloro-phenyl)-(4-methoxy-phenyl)-methyl]-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014780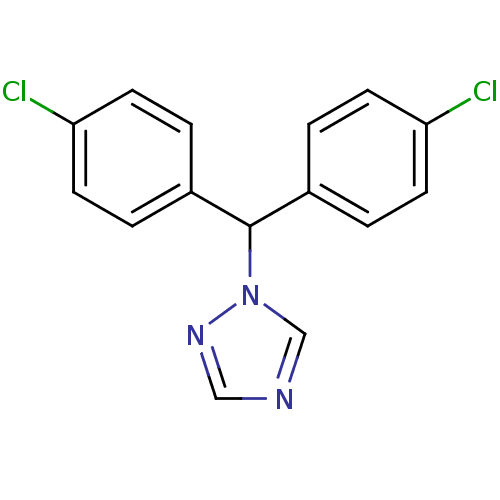 (1-[Bis-(4-chloro-phenyl)-methyl]-1H-[1,2,4]triazol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014773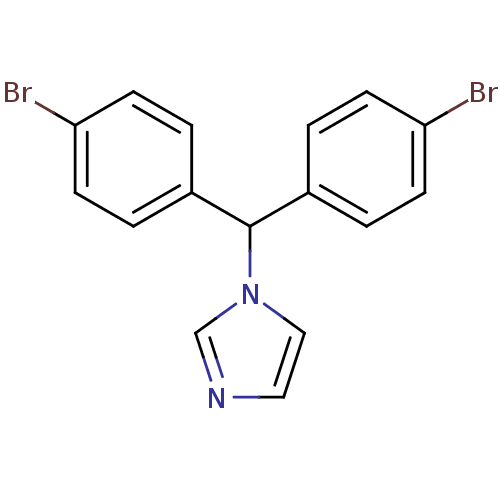 (1-[Bis-(4-bromo-phenyl)-methyl]-1H-imidazole | CHE...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014803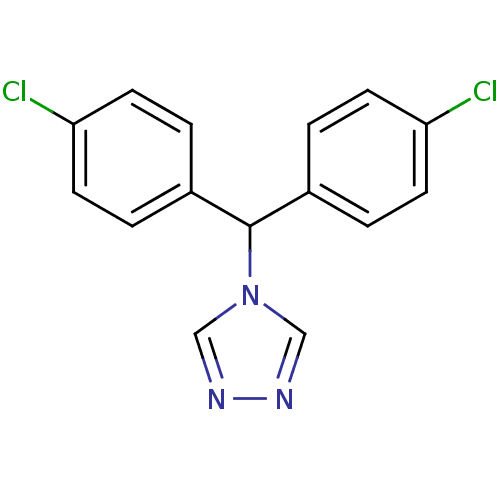 (4-[Bis-(4-chloro-phenyl)-methyl]-4H-[1,2,4]triazol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014776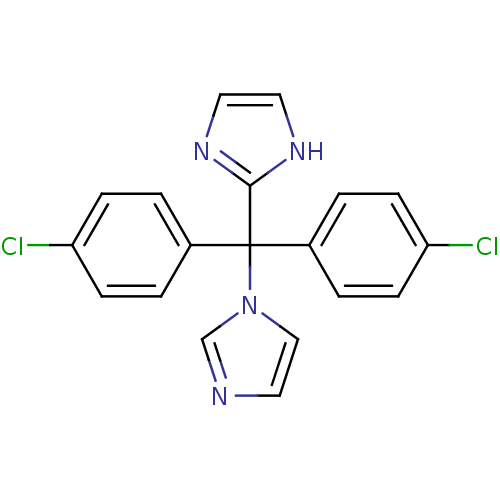 (1-[bis(4-chlorophenyl)(1H-imidazol-2-yl)methyl]-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014771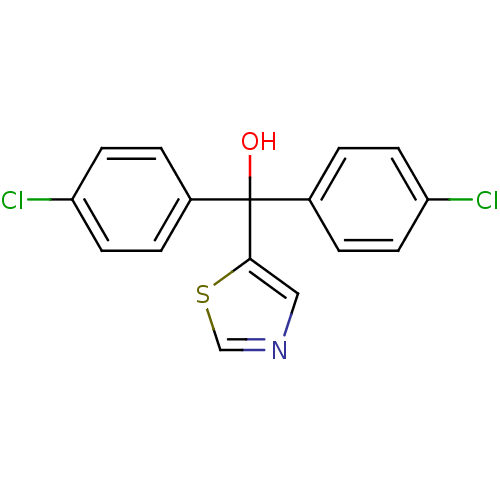 (Bis-(4-chloro-phenyl)-thiazol-5-yl-methanol | CHEM...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014764 (5-[Bis-(4-chloro-phenyl)-imidazol-1-yl-methyl]-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014809 (1-[(4-Chloro-phenyl)-(4-trifluoromethyl-phenyl)-me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014796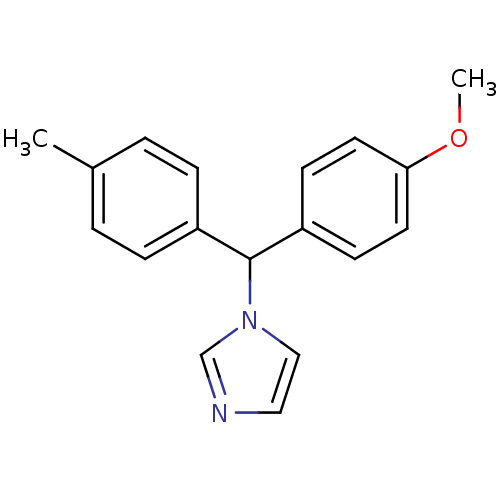 (1-[(4-Methoxy-phenyl)-p-tolyl-methyl]-1H-imidazole...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014763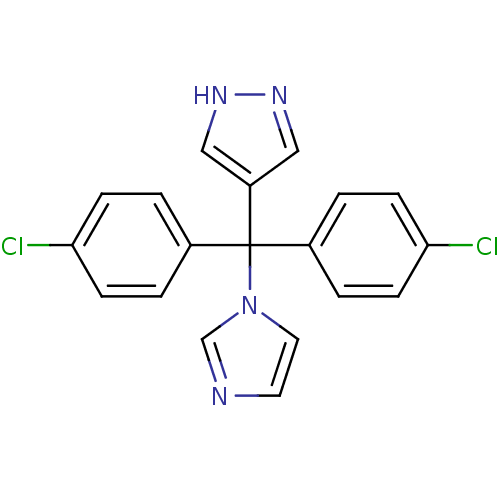 (4-[Bis-(4-chloro-phenyl)-imidazol-1-yl-methyl]-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014755 (1-[Bis-(4-methoxy-phenyl)-methyl]-1H-imidazole | C...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014800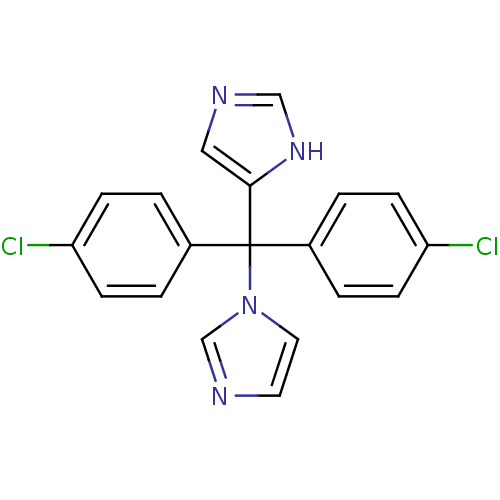 (1-[bis(4-chlorophenyl)(1H-imidazol-4-yl)methyl]-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014759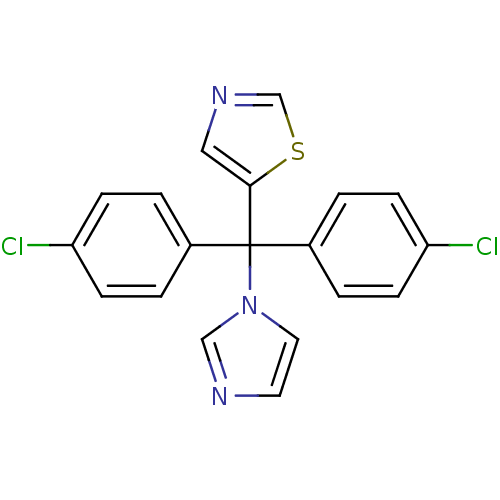 (5-[Bis-(4-chloro-phenyl)-imidazol-1-yl-methyl]-thi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014814 (4-[Bis-(4-chloro-phenyl)-imidazol-1-yl-methyl]-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014804 (Bis-(4-chloro-phenyl)-isothiazol-5-yl-methanol | C...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014782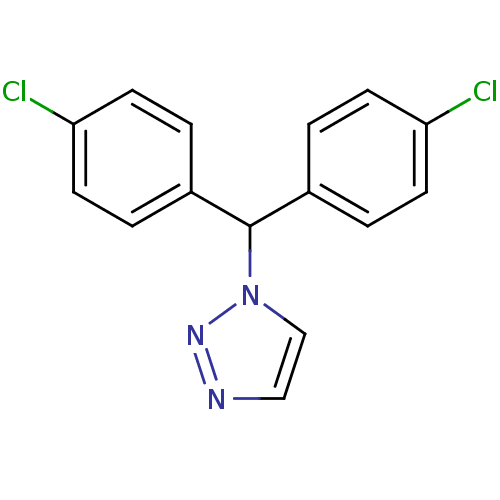 (1-[Bis-(4-chloro-phenyl)-methyl]-1H-[1,2,3]triazol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014808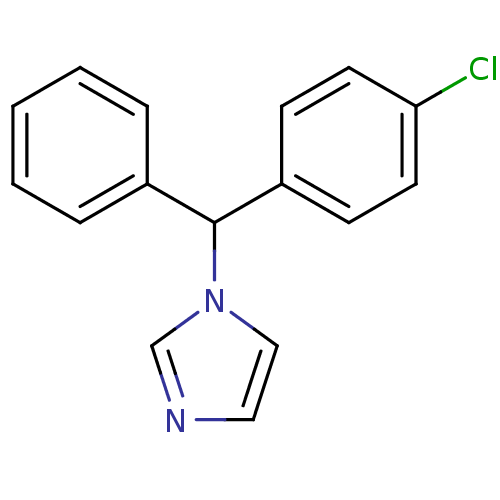 (1-[(4-Chloro-phenyl)-phenyl-methyl]-1H-imidazole |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014775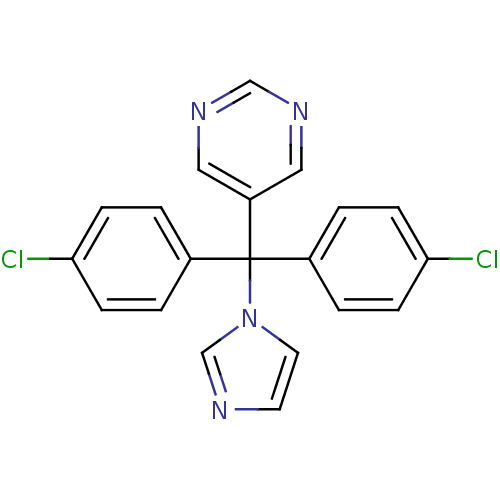 (5-[Bis-(4-chloro-phenyl)-imidazol-1-yl-methyl]-pyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014785 (Bis-(4-chloro-phenyl)-(1H-imidazol-4-yl)-methanol ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014783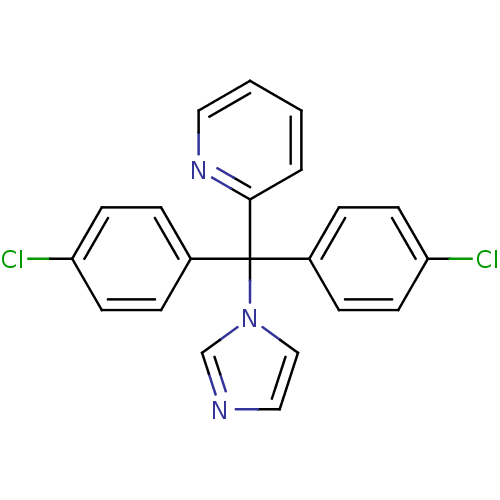 (2-[Bis-(4-chloro-phenyl)-imidazol-1-yl-methyl]-pyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014778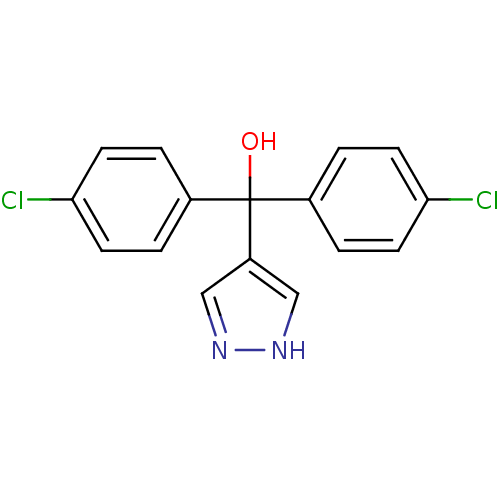 (Bis-(4-chloro-phenyl)-(1H-pyrazol-4-yl)-methanol |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014810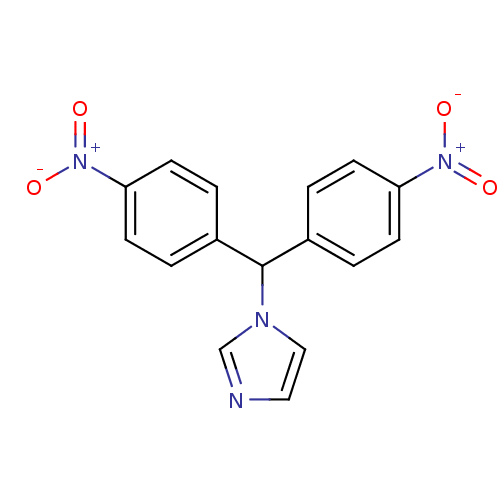 (1-[Bis-(4-nitro-phenyl)-methyl]-1H-imidazole | CHE...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014797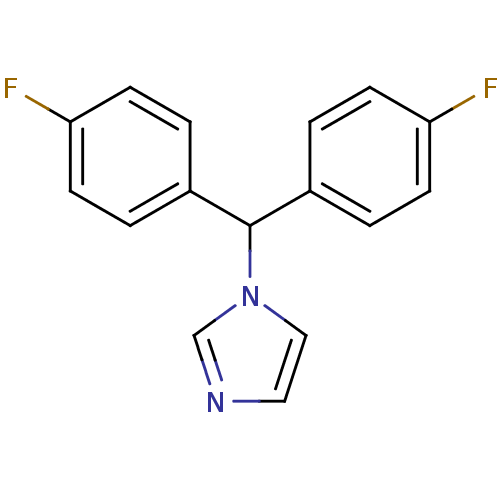 (1-[Bis-(4-fluoro-phenyl)-methyl]-1H-imidazole | CH...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014756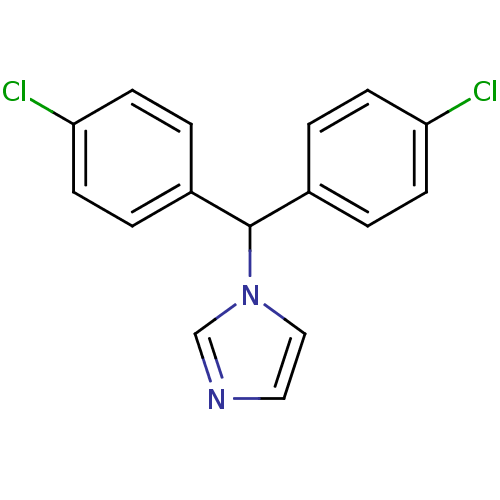 (1-[Bis-(4-chloro-phenyl)-methyl]-1H-imidazole | CH...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50367548 (CHEMBL1794921) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014750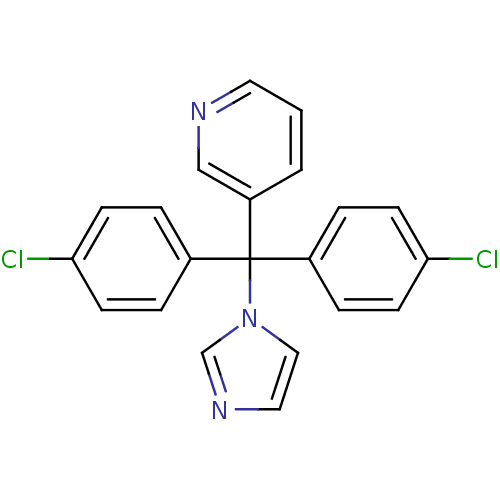 (3-[Bis-(4-chloro-phenyl)-imidazol-1-yl-methyl]-pyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014802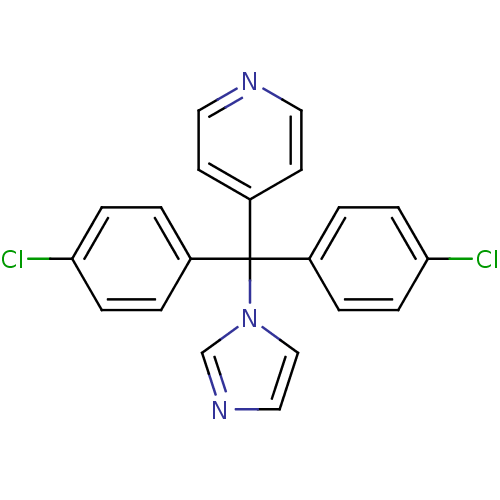 (4-[Bis-(4-chloro-phenyl)-imidazol-1-yl-methyl]-pyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014324 (5-[Bis-(4-chloro-phenyl)-methyl]-pyrimidine | 5-[B...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014324 (5-[Bis-(4-chloro-phenyl)-methyl]-pyrimidine | 5-[B...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014793 (5-[Bis-(4-chloro-phenyl)-imidazol-1-yl-methyl]-iso...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 57 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014749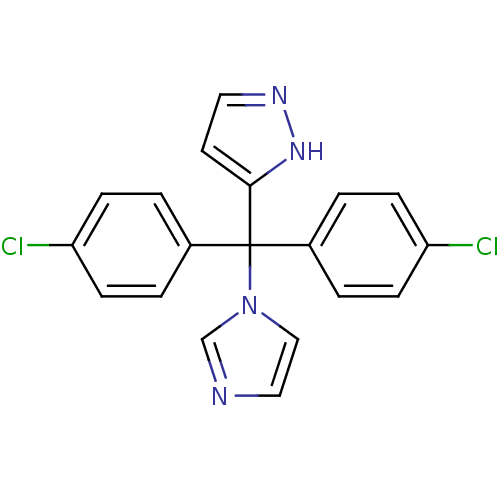 (3-[Bis-(4-chloro-phenyl)-imidazol-1-yl-methyl]-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014781 (1-[(2-Methoxy-phenyl)-phenyl-methyl]-1H-imidazole ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024481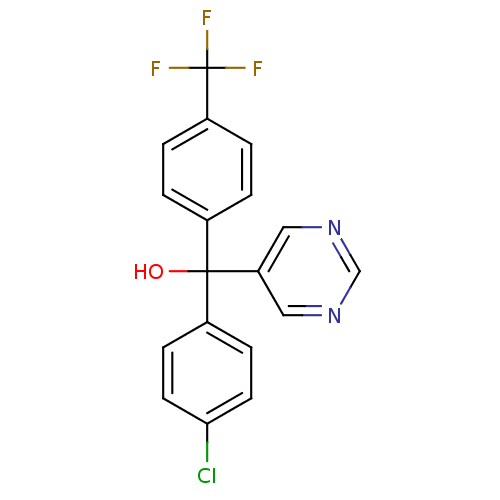 ((4-Chloro-phenyl)-pyrimidin-5-yl-(4-trifluoromethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014815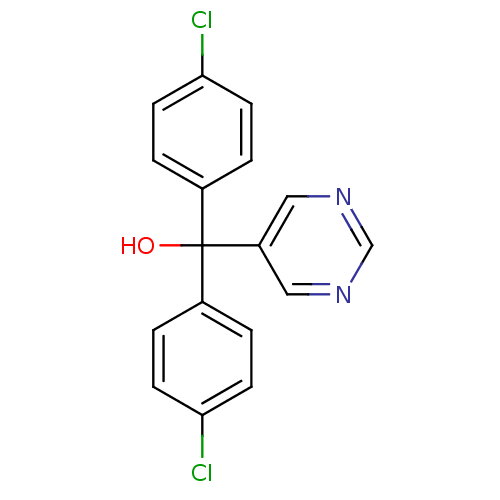 (Bis-(4-chloro-phenyl)-pyrimidin-5-yl-methanol | CH...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014815 (Bis-(4-chloro-phenyl)-pyrimidin-5-yl-methanol | CH...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014752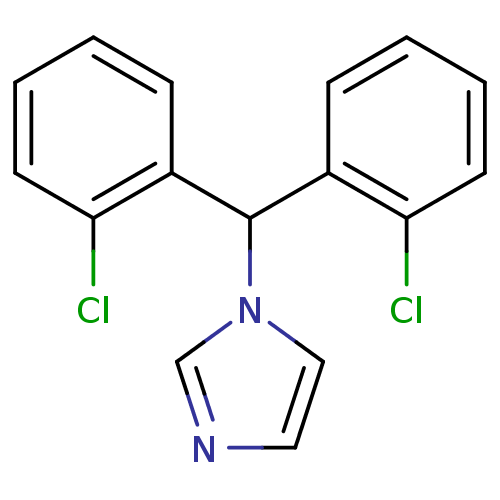 (1-[Bis-(2-chloro-phenyl)-methyl]-1H-imidazole | CH...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024516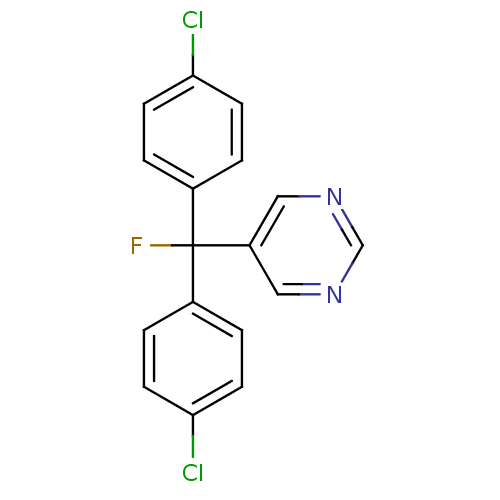 (5-[Bis-(4-chloro-phenyl)-fluoro-methyl]-pyrimidine...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024524 (5-[1,1-Bis-(4-chloro-phenyl)-ethyl]-pyrimidine | C...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 82 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014794 (3-[Bis-(4-chloro-phenyl)-methyl]-pyridine | CHEMBL...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014798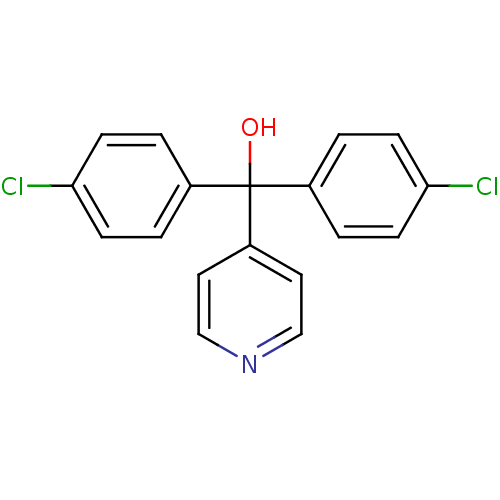 (Bis-(4-chloro-phenyl)-pyridin-4-yl-methanol | CHEM...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014791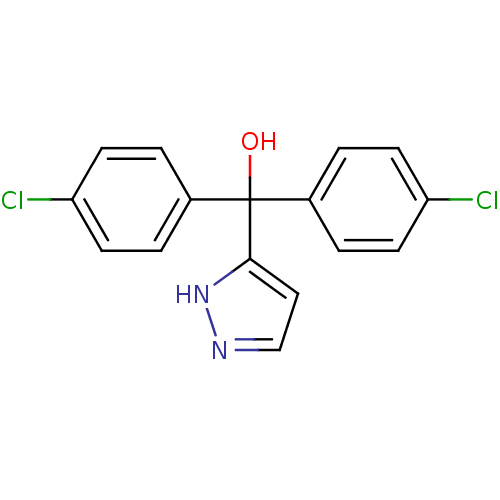 (Bis-(4-chloro-phenyl)-(1H-pyrazol-3-yl)-methanol |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014774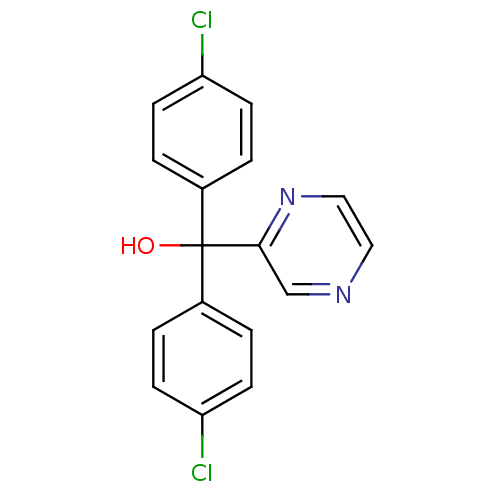 (Bis-(4-chloro-phenyl)-pyrazin-2-yl-methanol | CHEM...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 94 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014788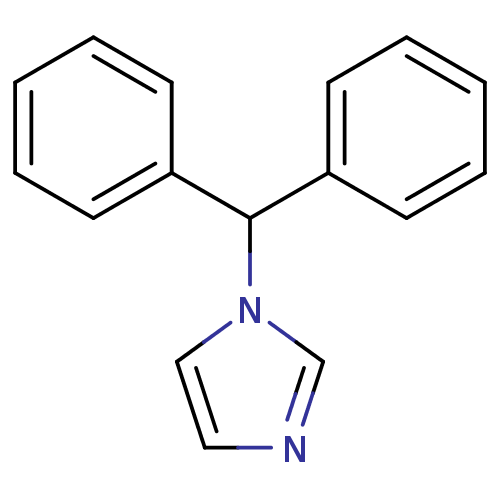 (1-Benzhydryl-1H-imidazole | CHEMBL336638) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 125 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024513 (6-Acetylsulfanyl-8-[2-[(4-amino-2-methyl-pyrimidin...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014751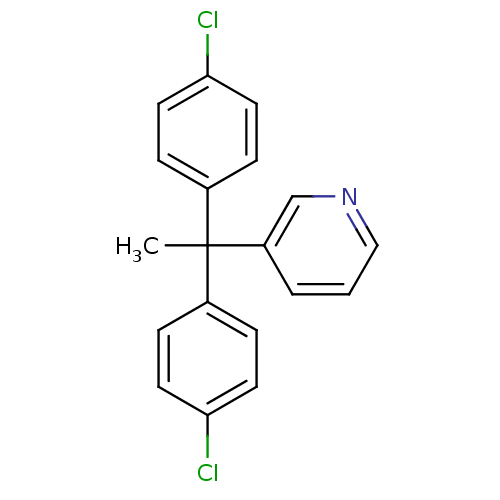 (3-[1,1-Bis-(4-chloro-phenyl)-ethyl]-pyridine | CHE...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 145 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014786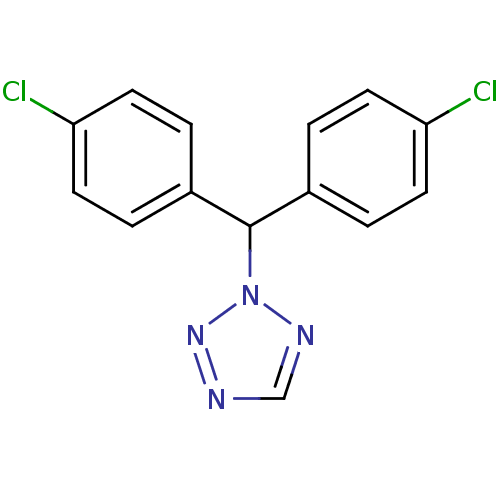 (2-[Bis-(4-chloro-phenyl)-methyl]-2H-tetrazole | CH...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014758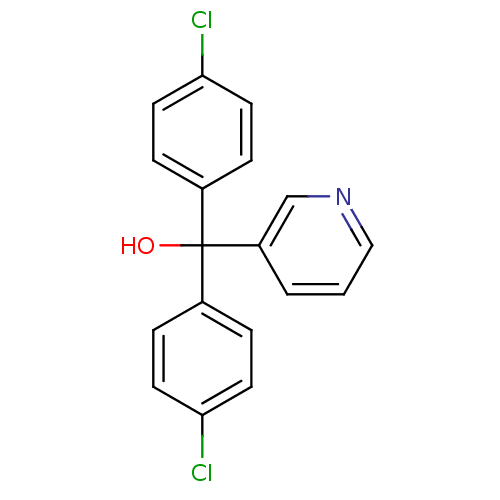 (Bis-(4-chloro-phenyl)-pyridin-3-yl-methanol | CHEM...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50014801 (4-[Bis-(4-chloro-phenyl)-methyl]-pyridine; hydroch...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Cytochrome P450 19A1 inhibition concentration to decrease aromatization of androstenedione in rat ovarian microsome | J Med Chem 33: 416-29 (1990) BindingDB Entry DOI: 10.7270/Q2WM1F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Rattus norvegicus) | BDBM50024522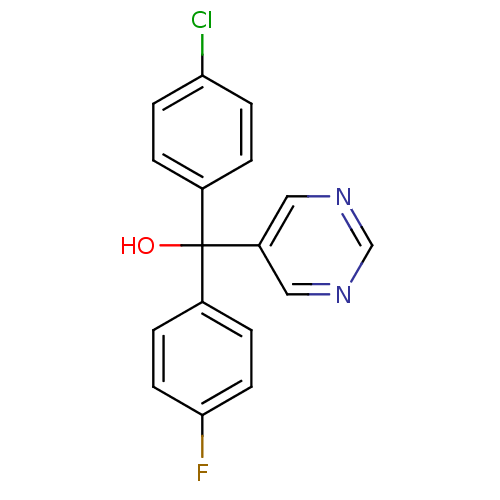 ((4-Chloro-phenyl)-(4-fluoro-phenyl)-pyrimidin-5-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of cytochrome P450 19A1 by rat ovarian microsomes incubated with [3H]androstenedione and NADPH-generating system. | J Med Chem 30: 1359-65 (1987) BindingDB Entry DOI: 10.7270/Q2NP251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 119 total ) | Next | Last >> |