 Found 208 hits of ec50 data for polymerid = 50000944
Found 208 hits of ec50 data for polymerid = 50000944 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50462106

(CHEMBL3736108)Show SMILES CC(C)C[C@H](NC(=O)c1c[nH]c2ccccc12)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C24H31N7O5/c1-13(2)10-18(30-20(32)15-11-28-16-7-4-3-6-14(15)16)22-31-19(12-36-22)21(33)29-17(23(34)35)8-5-9-27-24(25)26/h3-4,6-7,11-13,17-18,28H,5,8-10H2,1-2H3,(H,29,33)(H,30,32)(H,34,35)(H4,25,26,27)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50462106

(CHEMBL3736108)Show SMILES CC(C)C[C@H](NC(=O)c1c[nH]c2ccccc12)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C24H31N7O5/c1-13(2)10-18(30-20(32)15-11-28-16-7-4-3-6-14(15)16)22-31-19(12-36-22)21(33)29-17(23(34)35)8-5-9-27-24(25)26/h3-4,6-7,11-13,17-18,28H,5,8-10H2,1-2H3,(H,29,33)(H,30,32)(H,34,35)(H4,25,26,27)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in HMDM assessed as induction of Ca2+ release measured for 5 mins by Fluo-3 AM dye-based fluorescence assay |
J Med Chem 61: 3253-3276 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00882
BindingDB Entry DOI: 10.7270/Q2MK6GJ9 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50462106

(CHEMBL3736108)Show SMILES CC(C)C[C@H](NC(=O)c1c[nH]c2ccccc12)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C24H31N7O5/c1-13(2)10-18(30-20(32)15-11-28-16-7-4-3-6-14(15)16)22-31-19(12-36-22)21(33)29-17(23(34)35)8-5-9-27-24(25)26/h3-4,6-7,11-13,17-18,28H,5,8-10H2,1-2H3,(H,29,33)(H,30,32)(H,34,35)(H4,25,26,27)/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499185

(CHEMBL3736125)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)c1ccc(cc1)-c1ccccc1)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C31H38N6O6/c1-3-19(2)27(29-36-24(18-43-29)28(40)35-23(30(41)42)10-7-17-34-31(32)33)37-26(39)16-15-25(38)22-13-11-21(12-14-22)20-8-5-4-6-9-20/h4-6,8-9,11-14,18-19,23,27H,3,7,10,15-17H2,1-2H3,(H,35,40)(H,37,39)(H,41,42)(H4,32,33,34)/t19-,23-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499185

(CHEMBL3736125)Show SMILES CC[C@H](C)[C@H](NC(=O)CCC(=O)c1ccc(cc1)-c1ccccc1)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C31H38N6O6/c1-3-19(2)27(29-36-24(18-43-29)28(40)35-23(30(41)42)10-7-17-34-31(32)33)37-26(39)16-15-25(38)22-13-11-21(12-14-22)20-8-5-4-6-9-20/h4-6,8-9,11-14,18-19,23,27H,3,7,10,15-17H2,1-2H3,(H,35,40)(H,37,39)(H,41,42)(H4,32,33,34)/t19-,23-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50587350

(CHEMBL5076900)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human C3aR expressed in CHO cells assessed as induction of ERK1/2 phosphorylation at Thr202/Tyr204 residues incubated for 10 mins... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01174
BindingDB Entry DOI: 10.7270/Q2WS8Z5G |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50587347

(CHEMBL5081621)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human C3aR expressed in CHO cells assessed as induction of ERK1/2 phosphorylation at Thr202/Tyr204 residues incubated for 10 mins... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01174
BindingDB Entry DOI: 10.7270/Q2WS8Z5G |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50462105
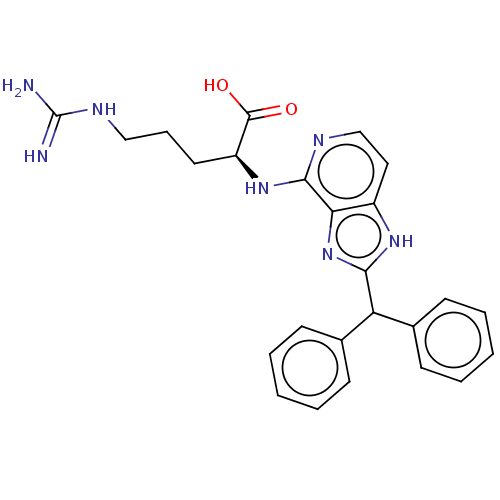
(CHEMBL4239035)Show SMILES NC(=N)NCCC[C@H](Nc1nccc2[nH]c(nc12)C(c1ccccc1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C25H27N7O2/c26-25(27)29-14-7-12-19(24(33)34)31-23-21-18(13-15-28-23)30-22(32-21)20(16-8-3-1-4-9-16)17-10-5-2-6-11-17/h1-6,8-11,13,15,19-20H,7,12,14H2,(H,28,31)(H,30,32)(H,33,34)(H4,26,27,29)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in HMDM assessed as induction of Ca2+ release |
J Med Chem 61: 3253-3276 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00882
BindingDB Entry DOI: 10.7270/Q2MK6GJ9 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499169
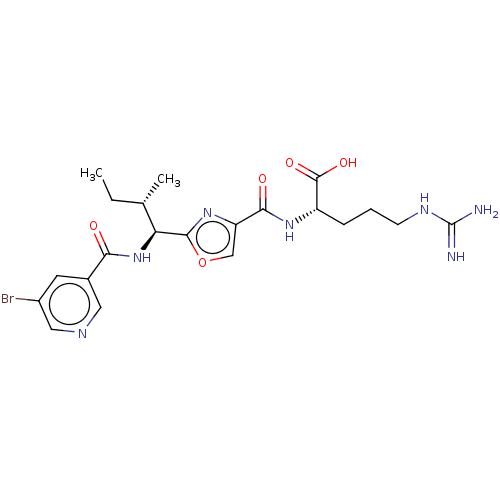
(CHEMBL3735507)Show SMILES CC[C@H](C)[C@H](NC(=O)c1cncc(Br)c1)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C21H28BrN7O5/c1-3-11(2)16(29-17(30)12-7-13(22)9-25-8-12)19-28-15(10-34-19)18(31)27-14(20(32)33)5-4-6-26-21(23)24/h7-11,14,16H,3-6H2,1-2H3,(H,27,31)(H,29,30)(H,32,33)(H4,23,24,26)/t11-,14-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499169

(CHEMBL3735507)Show SMILES CC[C@H](C)[C@H](NC(=O)c1cncc(Br)c1)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C21H28BrN7O5/c1-3-11(2)16(29-17(30)12-7-13(22)9-25-8-12)19-28-15(10-34-19)18(31)27-14(20(32)33)5-4-6-26-21(23)24/h7-11,14,16H,3-6H2,1-2H3,(H,27,31)(H,29,30)(H,32,33)(H4,23,24,26)/t11-,14-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499168

(CHEMBL3735760)Show SMILES CC(C)C[C@H](NC(=O)OC(C)(C)C)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C20H34N6O6/c1-11(2)9-13(26-19(30)32-20(3,4)5)16-25-14(10-31-16)15(27)24-12(17(28)29)7-6-8-23-18(21)22/h10-13H,6-9H2,1-5H3,(H,24,27)(H,26,30)(H,28,29)(H4,21,22,23)/t12-,13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499168

(CHEMBL3735760)Show SMILES CC(C)C[C@H](NC(=O)OC(C)(C)C)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C20H34N6O6/c1-11(2)9-13(26-19(30)32-20(3,4)5)16-25-14(10-31-16)15(27)24-12(17(28)29)7-6-8-23-18(21)22/h10-13H,6-9H2,1-5H3,(H,24,27)(H,26,30)(H,28,29)(H4,21,22,23)/t12-,13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50028696
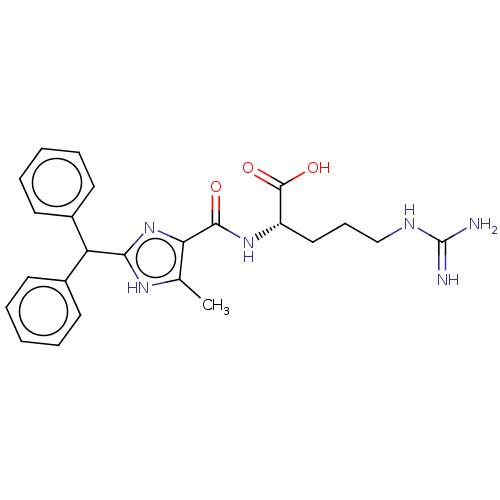
(CHEMBL3342689)Show SMILES Cc1[nH]c(nc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C24H28N6O3/c1-15-20(22(31)29-18(23(32)33)13-8-14-27-24(25)26)30-21(28-15)19(16-9-4-2-5-10-16)17-11-6-3-7-12-17/h2-7,9-12,18-19H,8,13-14H2,1H3,(H,28,30)(H,29,31)(H,32,33)(H4,25,26,27)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity against C3AR in HMDMs assessed as increase in intracellular calcium release by Fluo-3 AM dye based FLIPR assay relative to 1 uM C3a |
J Med Chem 57: 8459-70 (2014)
Article DOI: 10.1021/jm500956p
BindingDB Entry DOI: 10.7270/Q21R6S3N |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50028696

(CHEMBL3342689)Show SMILES Cc1[nH]c(nc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C24H28N6O3/c1-15-20(22(31)29-18(23(32)33)13-8-14-27-24(25)26)30-21(28-15)19(16-9-4-2-5-10-16)17-11-6-3-7-12-17/h2-7,9-12,18-19H,8,13-14H2,1H3,(H,28,30)(H,29,31)(H,32,33)(H4,25,26,27)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity against C3AR in HMDMs assessed as increase in intracellular calcium release by Fluo-3 AM dye based FLIPR assay relative to 1 uM C3a |
J Med Chem 57: 8459-70 (2014)
Article DOI: 10.1021/jm500956p
BindingDB Entry DOI: 10.7270/Q21R6S3N |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389001
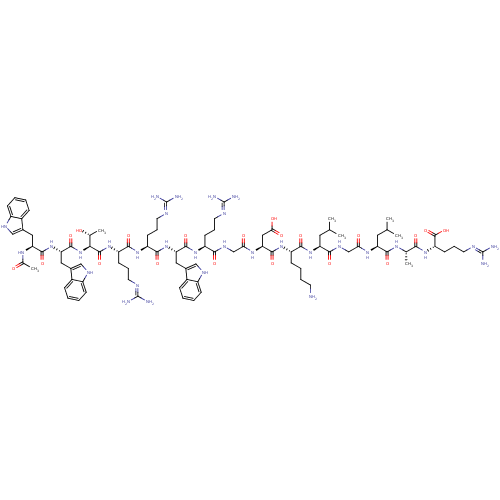
(CHEMBL2064016)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:106.122,88.90,121.128,66.75,41.48,29.33,12.16,4.4,132.137,wD:92.106,77.86,52.64,20.25,127.133,(46.53,-33.52,;46.54,-35.05,;47.87,-35.82,;45.2,-35.82,;45.2,-37.36,;43.87,-38.13,;42.54,-37.36,;42.54,-35.82,;41.21,-38.14,;39.87,-37.37,;38.53,-38.13,;38.54,-39.67,;37.2,-37.37,;37.2,-35.83,;38.54,-35.05,;38.54,-33.51,;39.87,-35.83,;35.87,-38.13,;34.54,-37.36,;34.53,-35.83,;33.2,-38.13,;33.2,-39.67,;34.53,-40.44,;34.54,-41.98,;35.87,-42.75,;35.87,-44.29,;31.87,-37.36,;30.54,-38.13,;30.54,-39.67,;29.2,-37.37,;29.2,-35.84,;30.53,-35.05,;31.86,-35.83,;30.53,-33.53,;27.87,-38.13,;26.53,-37.36,;26.53,-35.82,;25.2,-38.14,;23.87,-37.36,;22.54,-38.14,;22.54,-39.68,;21.2,-37.37,;21.2,-35.83,;22.53,-35.05,;22.53,-33.52,;23.86,-32.75,;23.86,-31.21,;22.53,-30.44,;25.2,-30.43,;19.87,-38.14,;18.54,-37.37,;18.54,-35.83,;17.2,-38.14,;17.2,-39.68,;18.54,-40.45,;19.94,-39.82,;20.97,-40.96,;20.21,-42.3,;20.68,-43.76,;19.65,-44.91,;18.15,-44.59,;17.67,-43.12,;18.7,-41.98,;15.87,-37.37,;14.54,-38.14,;14.54,-39.68,;13.21,-37.37,;13.21,-35.83,;14.53,-35.06,;14.53,-33.52,;15.87,-32.75,;15.86,-31.21,;14.53,-30.44,;17.2,-30.44,;11.87,-38.14,;10.53,-37.37,;10.53,-35.83,;9.2,-38.15,;9.2,-39.68,;10.54,-40.45,;10.54,-41.99,;11.87,-42.76,;11.87,-44.3,;10.54,-45.07,;13.2,-45.07,;7.87,-37.37,;6.53,-38.14,;6.53,-39.68,;5.2,-37.37,;3.87,-38.14,;2.54,-37.37,;2.54,-35.83,;1.2,-38.15,;1.21,-39.69,;2.54,-40.45,;3.94,-39.83,;4.97,-40.97,;4.2,-42.3,;4.68,-43.77,;3.65,-44.91,;2.15,-44.6,;1.67,-43.13,;2.69,-41.98,;-.13,-37.37,;-1.47,-38.15,;-1.47,-39.69,;-2.8,-37.38,;-2.8,-35.84,;-1.47,-35.06,;-.06,-35.7,;.97,-34.55,;.2,-33.21,;.67,-31.75,;-.36,-30.61,;-1.86,-30.93,;-2.34,-32.39,;-1.31,-33.54,;-4.13,-38.14,;-5.46,-37.38,;-6.78,-38.15,;-5.46,-35.84,;5.2,-35.84,;3.87,-35.06,;6.54,-35.06,;46.53,-38.13,;46.53,-39.67,;47.87,-37.36,;49.21,-38.13,;49.2,-39.67,;50.54,-37.35,;50.53,-35.83,;51.87,-38.13,;53.21,-37.35,;53.21,-35.82,;54.53,-35.05,;54.53,-33.51,;55.87,-32.74,;55.87,-31.19,;54.53,-30.43,;57.2,-30.43,;54.54,-38.14,;55.87,-37.36,;54.53,-39.66,)| Show InChI InChI=1S/C92H139N31O20/c1-47(2)36-66(78(132)110-46-72(126)113-67(37-48(3)4)82(136)111-49(5)76(130)119-65(88(142)143)30-19-35-105-92(100)101)120-80(134)62(26-14-15-31-93)117-85(139)71(41-74(128)129)114-73(127)45-109-77(131)61(27-16-32-102-89(94)95)115-84(138)69(39-53-43-107-59-24-12-9-21-56(53)59)121-81(135)63(28-17-33-103-90(96)97)116-79(133)64(29-18-34-104-91(98)99)118-87(141)75(50(6)124)123-86(140)70(40-54-44-108-60-25-13-10-22-57(54)60)122-83(137)68(112-51(7)125)38-52-42-106-58-23-11-8-20-55(52)58/h8-13,20-25,42-44,47-50,61-71,75,106-108,124H,14-19,26-41,45-46,93H2,1-7H3,(H,109,131)(H,110,132)(H,111,136)(H,112,125)(H,113,126)(H,114,127)(H,115,138)(H,116,133)(H,117,139)(H,118,141)(H,119,130)(H,120,134)(H,121,135)(H,122,137)(H,123,140)(H,128,129)(H,142,143)(H4,94,95,102)(H4,96,97,103)(H4,98,99,104)(H4,100,101,105)/t49-,50+,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,75-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Agonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant by degranulation assay |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499172

(CHEMBL3736239)Show SMILES CC[C@H](C)[C@H](NC(=O)OC(C)(C)C)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C20H34N6O6/c1-6-11(2)14(26-19(30)32-20(3,4)5)16-25-13(10-31-16)15(27)24-12(17(28)29)8-7-9-23-18(21)22/h10-12,14H,6-9H2,1-5H3,(H,24,27)(H,26,30)(H,28,29)(H4,21,22,23)/t11-,12-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499172

(CHEMBL3736239)Show SMILES CC[C@H](C)[C@H](NC(=O)OC(C)(C)C)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C20H34N6O6/c1-6-11(2)14(26-19(30)32-20(3,4)5)16-25-13(10-31-16)15(27)24-12(17(28)29)8-7-9-23-18(21)22/h10-12,14H,6-9H2,1-5H3,(H,24,27)(H,26,30)(H,28,29)(H4,21,22,23)/t11-,12-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50388995
(CHEMBL2064019)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:110.126,87.94,65.74,40.47,29.31,12.16,4.4,133.138,wD:96.110,76.85,51.63,35.35,20.25,128.134,(43.19,-33.09,;43.2,-34.64,;44.54,-35.39,;41.87,-35.41,;41.88,-36.95,;40.55,-37.72,;39.21,-36.96,;39.2,-35.43,;37.89,-37.74,;36.55,-36.97,;35.21,-37.75,;35.22,-39.29,;33.88,-36.99,;33.87,-35.45,;35.2,-34.67,;35.19,-33.14,;36.54,-35.43,;32.55,-37.77,;31.21,-37,;31.2,-35.46,;29.88,-37.78,;29.89,-39.32,;31.22,-40.08,;31.23,-41.62,;32.57,-42.39,;32.58,-43.93,;28.54,-37.02,;27.21,-37.79,;27.22,-39.33,;25.87,-37.03,;25.87,-35.49,;27.19,-34.71,;24.54,-37.81,;23.2,-37.04,;23.2,-35.51,;21.88,-37.82,;21.88,-39.36,;20.54,-37.06,;19.21,-37.84,;19.22,-39.38,;17.87,-37.08,;17.86,-35.54,;19.19,-34.75,;19.18,-33.22,;20.51,-32.44,;20.5,-30.9,;19.16,-30.14,;21.83,-30.13,;16.55,-37.85,;15.21,-37.09,;15.2,-35.55,;13.87,-37.87,;13.88,-39.41,;15.22,-40.17,;16.62,-39.54,;17.66,-40.67,;16.9,-42.01,;17.38,-43.47,;16.36,-44.63,;14.85,-44.31,;14.36,-42.85,;15.39,-41.7,;12.54,-37.1,;11.21,-37.88,;11.22,-39.42,;9.87,-37.12,;9.86,-35.58,;11.19,-34.8,;11.18,-33.26,;12.51,-32.48,;12.5,-30.94,;11.16,-30.18,;13.84,-30.16,;8.54,-37.89,;7.2,-37.13,;7.19,-35.59,;5.87,-37.91,;5.88,-39.45,;7.22,-40.21,;7.23,-41.75,;8.56,-42.52,;8.57,-44.06,;7.24,-44.83,;9.91,-44.82,;4.53,-37.15,;3.2,-37.92,;3.21,-39.46,;1.86,-37.16,;1.85,-35.62,;3.19,-34.84,;3.18,-33.31,;1.84,-32.54,;4.51,-32.52,;.54,-37.94,;-.8,-37.17,;-.81,-35.63,;-2.13,-37.96,;-2.12,-39.5,;-.78,-40.25,;.62,-39.62,;1.65,-40.76,;.89,-42.1,;1.37,-43.56,;.35,-44.71,;-1.15,-44.4,;-1.64,-42.94,;-.62,-41.79,;-3.46,-37.19,;-4.79,-37.97,;-4.79,-39.51,;-6.14,-37.2,;-6.15,-35.67,;-4.82,-34.88,;-3.41,-35.5,;-2.38,-34.36,;-3.16,-33.02,;-2.69,-31.55,;-3.73,-30.42,;-5.23,-30.75,;-5.7,-32.21,;-4.67,-33.35,;-7.47,-37.98,;-8.8,-37.22,;-10.12,-37.99,;-8.8,-35.68,;43.22,-37.71,;43.22,-39.24,;44.55,-36.94,;45.89,-37.7,;45.89,-39.24,;47.21,-36.92,;47.2,-35.38,;48.55,-37.68,;49.88,-36.91,;49.88,-35.36,;51.2,-34.59,;51.19,-33.05,;52.52,-32.26,;52.51,-30.74,;51.18,-29.97,;53.85,-29.95,;51.22,-37.67,;52.55,-36.89,;51.23,-39.21,)| Show InChI InChI=1S/C93H141N31O20/c1-48(2)38-68(78(132)110-46-74(127)114-69(39-49(3)4)84(138)112-50(5)76(130)120-67(89(143)144)30-19-37-106-93(101)102)121-81(135)62(26-14-15-33-94)118-88(142)73(47-125)124-77(131)51(6)111-79(133)63(27-16-34-103-90(95)96)117-86(140)71(41-54-44-108-60-24-12-9-21-57(54)60)122-82(136)65(29-18-36-105-92(99)100)115-80(134)64(28-17-35-104-91(97)98)116-83(137)66(31-32-75(128)129)119-87(141)72(42-55-45-109-61-25-13-10-22-58(55)61)123-85(139)70(113-52(7)126)40-53-43-107-59-23-11-8-20-56(53)59/h8-13,20-25,43-45,48-51,62-73,107-109,125H,14-19,26-42,46-47,94H2,1-7H3,(H,110,132)(H,111,133)(H,112,138)(H,113,126)(H,114,127)(H,115,134)(H,116,137)(H,117,140)(H,118,142)(H,119,141)(H,120,130)(H,121,135)(H,122,136)(H,123,139)(H,124,131)(H,128,129)(H,143,144)(H4,95,96,103)(H4,97,98,104)(H4,99,100,105)(H4,101,102,106)/t50-,51-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 35.5 | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Agonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant by degranulation assay |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50587351
(CHEMBL5080234)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human C3aR expressed in CHO cells assessed as induction of ERK1/2 phosphorylation at Thr202/Tyr204 residues incubated for 10 mins... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01174
BindingDB Entry DOI: 10.7270/Q2WS8Z5G |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50028697

(CHEMBL3342690)Show SMILES Cc1c(nc(C(c2ccccc2)c2ccccc2)n1C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C25H30N6O3/c1-16-21(23(32)29-19(24(33)34)14-9-15-28-25(26)27)30-22(31(16)2)20(17-10-5-3-6-11-17)18-12-7-4-8-13-18/h3-8,10-13,19-20H,9,14-15H2,1-2H3,(H,29,32)(H,33,34)(H4,26,27,28)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity against C3AR in HMDMs assessed as increase in intracellular calcium release by Fluo-3 AM dye based FLIPR assay relative to 1 uM C3a |
J Med Chem 57: 8459-70 (2014)
Article DOI: 10.1021/jm500956p
BindingDB Entry DOI: 10.7270/Q21R6S3N |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50028697

(CHEMBL3342690)Show SMILES Cc1c(nc(C(c2ccccc2)c2ccccc2)n1C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C25H30N6O3/c1-16-21(23(32)29-19(24(33)34)14-9-15-28-25(26)27)30-22(31(16)2)20(17-10-5-3-6-11-17)18-12-7-4-8-13-18/h3-8,10-13,19-20H,9,14-15H2,1-2H3,(H,29,32)(H,33,34)(H4,26,27,28)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity against C3AR in HMDMs assessed as increase in intracellular calcium release by Fluo-3 AM dye based FLIPR assay relative to 1 uM C3a |
J Med Chem 57: 8459-70 (2014)
Article DOI: 10.1021/jm500956p
BindingDB Entry DOI: 10.7270/Q21R6S3N |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499166

(CHEMBL3735048)Show SMILES CC[C@H](C)[C@H](NC(=O)c1ccc2ccccc2n1)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C25H31N7O5/c1-3-14(2)20(32-21(33)17-11-10-15-7-4-5-8-16(15)29-17)23-31-19(13-37-23)22(34)30-18(24(35)36)9-6-12-28-25(26)27/h4-5,7-8,10-11,13-14,18,20H,3,6,9,12H2,1-2H3,(H,30,34)(H,32,33)(H,35,36)(H4,26,27,28)/t14-,18-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499166

(CHEMBL3735048)Show SMILES CC[C@H](C)[C@H](NC(=O)c1ccc2ccccc2n1)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C25H31N7O5/c1-3-14(2)20(32-21(33)17-11-10-15-7-4-5-8-16(15)29-17)23-31-19(13-37-23)22(34)30-18(24(35)36)9-6-12-28-25(26)27/h4-5,7-8,10-11,13-14,18,20H,3,6,9,12H2,1-2H3,(H,30,34)(H,32,33)(H,35,36)(H4,26,27,28)/t14-,18-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499171

(CHEMBL3735339)Show SMILES CC(C)C[C@H](NC(=O)OC(C)(C)C)c1nc(C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)c(C)o1 |r| Show InChI InChI=1S/C21H36N6O6/c1-11(2)10-14(26-20(31)33-21(4,5)6)17-27-15(12(3)32-17)16(28)25-13(18(29)30)8-7-9-24-19(22)23/h11,13-14H,7-10H2,1-6H3,(H,25,28)(H,26,31)(H,29,30)(H4,22,23,24)/t13-,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520316
(CHEMBL4445758)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1cccc(Cl)c1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H26Cl2N4O3S/c1-14-11-20(21(15-5-2-7-17(26)12-15)16-6-3-8-18(27)13-16)35-22(14)23(32)31-19(24(33)34)9-4-10-30-25(28)29/h2-3,5-8,11-13,19,21H,4,9-10H2,1H3,(H,31,32)(H,33,34)(H4,28,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at C3a receptor in human MDM cells assessed as induction of intracellular calcium release measured at 1 sec interval for => 200 secs... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520316

(CHEMBL4445758)Show SMILES Cc1cc(sc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(c1cccc(Cl)c1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H26Cl2N4O3S/c1-14-11-20(21(15-5-2-7-17(26)12-15)16-6-3-8-18(27)13-16)35-22(14)23(32)31-19(24(33)34)9-4-10-30-25(28)29/h2-3,5-8,11-13,19,21H,4,9-10H2,1H3,(H,31,32)(H,33,34)(H4,28,29,30)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at C3a receptor in human MDM cells assessed as induction of intracellular calcium release measured at 1 sec interval for => 200 secs... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520303
(CHEMBL4459830)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(c1cccc(Cl)c1)c1cccc(Cl)c1)C(O)=O |r| Show InChI InChI=1S/C24H24Cl2N4O3S/c25-16-6-1-4-14(12-16)21(15-5-2-7-17(26)13-15)19-9-10-20(34-19)22(31)30-18(23(32)33)8-3-11-29-24(27)28/h1-2,4-7,9-10,12-13,18,21H,3,8,11H2,(H,30,31)(H,32,33)(H4,27,28,29)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at C3a receptor in human MDM cells assessed as induction of intracellular calcium release measured at 1 sec interval for => 200 secs... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520303

(CHEMBL4459830)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(c1cccc(Cl)c1)c1cccc(Cl)c1)C(O)=O |r| Show InChI InChI=1S/C24H24Cl2N4O3S/c25-16-6-1-4-14(12-16)21(15-5-2-7-17(26)13-15)19-9-10-20(34-19)22(31)30-18(23(32)33)8-3-11-29-24(27)28/h1-2,4-7,9-10,12-13,18,21H,3,8,11H2,(H,30,31)(H,32,33)(H4,27,28,29)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at C3a receptor in human MDM cells assessed as induction of intracellular calcium release measured at 1 sec interval for => 200 secs... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499183
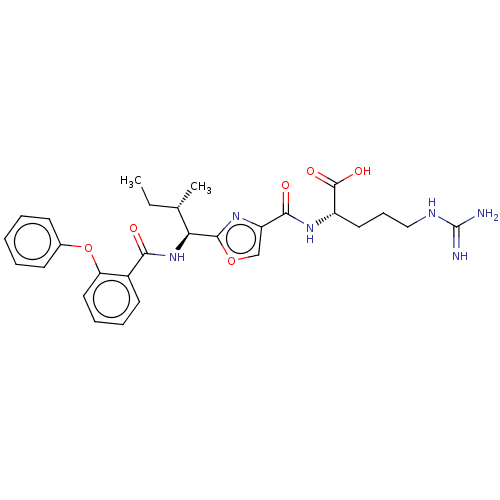
(CHEMBL3735764)Show SMILES CC[C@H](C)[C@H](NC(=O)c1ccccc1Oc1ccccc1)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C28H34N6O6/c1-3-17(2)23(34-24(35)19-12-7-8-14-22(19)40-18-10-5-4-6-11-18)26-33-21(16-39-26)25(36)32-20(27(37)38)13-9-15-31-28(29)30/h4-8,10-12,14,16-17,20,23H,3,9,13,15H2,1-2H3,(H,32,36)(H,34,35)(H,37,38)(H4,29,30,31)/t17-,20-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499183

(CHEMBL3735764)Show SMILES CC[C@H](C)[C@H](NC(=O)c1ccccc1Oc1ccccc1)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C28H34N6O6/c1-3-17(2)23(34-24(35)19-12-7-8-14-22(19)40-18-10-5-4-6-11-18)26-33-21(16-39-26)25(36)32-20(27(37)38)13-9-15-31-28(29)30/h4-8,10-12,14,16-17,20,23H,3,9,13,15H2,1-2H3,(H,32,36)(H,34,35)(H,37,38)(H4,29,30,31)/t17-,20-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50587346
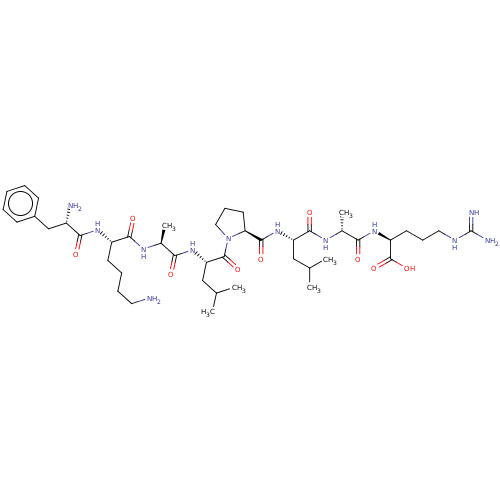
(CHEMBL5085196)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human C3aR expressed in CHO cells assessed as induction of ERK1/2 phosphorylation at Thr202/Tyr204 residues incubated for 10 mins... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01174
BindingDB Entry DOI: 10.7270/Q2WS8Z5G |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520305

(CHEMBL4559976)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(C1CCCCC1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H32N4O3S/c25-24(26)27-15-7-12-18(23(30)31)28-22(29)20-14-13-19(32-20)21(16-8-3-1-4-9-16)17-10-5-2-6-11-17/h1,3-4,8-9,13-14,17-18,21H,2,5-7,10-12,15H2,(H,28,29)(H,30,31)(H4,25,26,27)/t18-,21?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at C3a receptor in human MDM cells assessed as induction of intracellular calcium release measured at 1 sec interval for => 200 secs... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50520305

(CHEMBL4559976)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccc(s1)C(C1CCCCC1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H32N4O3S/c25-24(26)27-15-7-12-18(23(30)31)28-22(29)20-14-13-19(32-20)21(16-8-3-1-4-9-16)17-10-5-2-6-11-17/h1,3-4,8-9,13-14,17-18,21H,2,5-7,10-12,15H2,(H,28,29)(H,30,31)(H4,25,26,27)/t18-,21?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at C3a receptor in human MDM cells assessed as induction of intracellular calcium release measured at 1 sec interval for => 200 secs... |
J Med Chem 63: 529-541 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00927
BindingDB Entry DOI: 10.7270/Q2377D3X |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389002
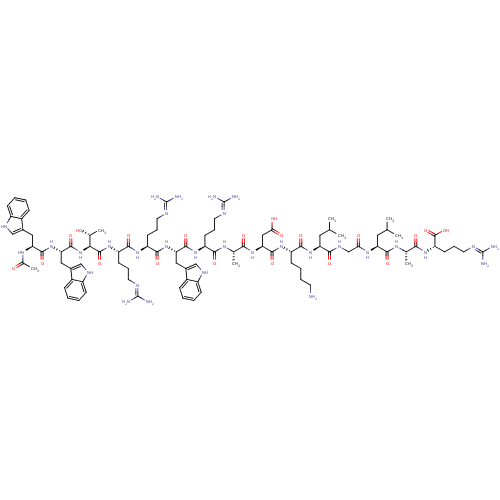
(CHEMBL2064017)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:107.123,89.91,122.129,67.76,42.49,29.33,12.16,4.4,133.138,wD:93.107,78.87,53.65,37.37,20.25,128.134,(51.04,2.49,;51.04,.95,;52.38,.19,;49.71,.18,;49.71,-1.36,;48.38,-2.14,;47.04,-1.38,;47.04,.17,;45.72,-2.15,;44.38,-1.37,;43.04,-2.16,;43.05,-3.68,;41.71,-1.38,;41.71,.15,;43.04,.92,;43.04,2.46,;44.37,.15,;40.38,-2.15,;39.04,-1.39,;39.04,.15,;37.71,-2.16,;37.71,-3.7,;39.05,-4.47,;39.05,-6.01,;40.39,-6.77,;40.39,-8.31,;36.37,-1.39,;35.04,-2.17,;35.05,-3.71,;33.71,-1.41,;33.71,.14,;35.03,.91,;36.37,.15,;35.03,2.45,;32.37,-2.18,;31.04,-1.41,;31.03,.13,;29.71,-2.18,;29.71,-3.72,;28.37,-1.41,;27.05,-2.19,;27.05,-3.73,;25.71,-1.42,;25.71,.11,;27.03,.88,;27.03,2.43,;28.36,3.21,;28.36,4.75,;27.02,5.51,;29.69,5.52,;24.38,-2.19,;23.04,-1.43,;23.04,.12,;21.71,-2.2,;21.71,-3.74,;23.05,-4.51,;24.45,-3.87,;25.49,-5.02,;24.72,-6.35,;25.2,-7.82,;24.18,-8.97,;22.67,-8.65,;22.19,-7.19,;23.22,-6.04,;20.38,-1.43,;19.05,-2.21,;19.05,-3.75,;17.71,-1.44,;17.71,.1,;19.04,.87,;19.03,2.4,;20.36,3.17,;20.36,4.72,;19.02,5.49,;21.69,5.49,;16.38,-2.21,;15.04,-1.45,;15.04,.09,;13.71,-2.22,;13.72,-3.76,;15.05,-4.53,;15.05,-6.07,;16.39,-6.83,;16.39,-8.37,;15.06,-9.14,;17.73,-9.14,;12.37,-1.45,;11.04,-2.23,;11.04,-3.76,;9.7,-1.45,;8.38,-2.23,;7.04,-1.46,;7.04,.07,;5.71,-2.24,;5.71,-3.78,;7.05,-4.54,;8.45,-3.92,;9.49,-5.06,;8.72,-6.39,;9.2,-7.86,;8.17,-9.01,;6.67,-8.69,;6.19,-7.22,;7.21,-6.08,;4.38,-1.47,;3.05,-2.25,;3.05,-3.79,;1.71,-1.48,;1.7,.07,;3.03,.84,;4.44,.21,;5.47,1.36,;4.7,2.69,;5.17,4.16,;4.13,5.3,;2.63,4.97,;2.15,3.51,;3.2,2.37,;.39,-2.25,;-.94,-1.49,;-2.27,-2.26,;-.95,.05,;9.7,.08,;8.36,.85,;11.04,.85,;51.04,-2.13,;51.05,-3.67,;52.38,-1.36,;53.72,-2.12,;53.72,-3.67,;55.04,-1.35,;55.04,.19,;56.38,-2.11,;57.71,-1.34,;57.7,.19,;59.04,.97,;59.04,2.51,;60.36,3.28,;60.36,4.82,;59.03,5.59,;61.7,5.6,;59.05,-2.11,;60.38,-1.33,;59.05,-3.65,)| Show InChI InChI=1S/C93H141N31O20/c1-47(2)37-67(78(132)110-46-73(127)114-68(38-48(3)4)83(137)112-49(5)76(130)119-66(89(143)144)31-20-36-106-93(101)102)121-81(135)62(27-15-16-32-94)116-86(140)72(42-74(128)129)120-77(131)50(6)111-79(133)63(28-17-33-103-90(95)96)117-85(139)70(40-54-44-108-60-25-13-10-22-57(54)60)122-82(136)64(29-18-34-104-91(97)98)115-80(134)65(30-19-35-105-92(99)100)118-88(142)75(51(7)125)124-87(141)71(41-55-45-109-61-26-14-11-23-58(55)61)123-84(138)69(113-52(8)126)39-53-43-107-59-24-12-9-21-56(53)59/h9-14,21-26,43-45,47-51,62-72,75,107-109,125H,15-20,27-42,46,94H2,1-8H3,(H,110,132)(H,111,133)(H,112,137)(H,113,126)(H,114,127)(H,115,134)(H,116,140)(H,117,139)(H,118,142)(H,119,130)(H,120,131)(H,121,135)(H,122,136)(H,123,138)(H,124,141)(H,128,129)(H,143,144)(H4,95,96,103)(H4,97,98,104)(H4,99,100,105)(H4,101,102,106)/t49-,50-,51+,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,75-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 66.1 | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Agonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant by degranulation assay |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499184

(CHEMBL3735993)Show SMILES CC[C@H](C)[C@H](NC(=O)Cc1c[nH]c2ccccc12)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C25H33N7O5/c1-3-14(2)21(32-20(33)11-15-12-29-17-8-5-4-7-16(15)17)23-31-19(13-37-23)22(34)30-18(24(35)36)9-6-10-28-25(26)27/h4-5,7-8,12-14,18,21,29H,3,6,9-11H2,1-2H3,(H,30,34)(H,32,33)(H,35,36)(H4,26,27,28)/t14-,18-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499184

(CHEMBL3735993)Show SMILES CC[C@H](C)[C@H](NC(=O)Cc1c[nH]c2ccccc12)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C25H33N7O5/c1-3-14(2)21(32-20(33)11-15-12-29-17-8-5-4-7-16(15)17)23-31-19(13-37-23)22(34)30-18(24(35)36)9-6-10-28-25(26)27/h4-5,7-8,12-14,18,21,29H,3,6,9-11H2,1-2H3,(H,30,34)(H,32,33)(H,35,36)(H4,26,27,28)/t14-,18-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389004
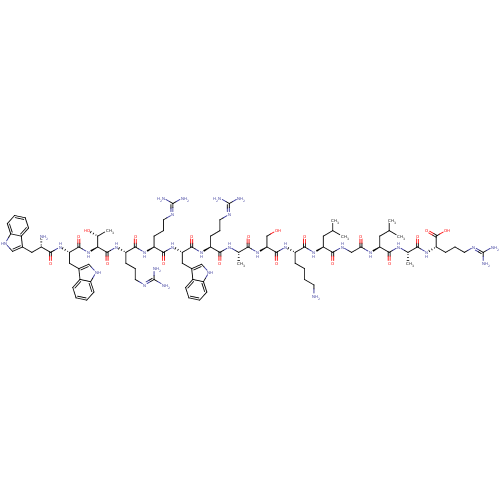
(CHEMBL2064021)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:105.109,87.89,117.124,65.74,40.47,29.31,12.16,4.4,128.133,wD:91.105,76.85,51.63,35.35,20.25,123.129,(43.63,-17.18,;43.63,-18.73,;44.96,-19.49,;42.29,-19.5,;42.29,-21.03,;40.96,-21.8,;39.62,-21.03,;39.62,-19.49,;38.29,-21.8,;36.96,-21.02,;35.62,-21.8,;35.62,-23.34,;34.28,-21.02,;34.29,-19.48,;35.63,-18.71,;35.63,-17.18,;36.96,-19.49,;32.96,-21.8,;31.62,-21.02,;31.62,-19.48,;30.28,-21.79,;30.29,-23.32,;31.61,-24.1,;31.61,-25.64,;32.95,-26.41,;32.94,-27.95,;28.95,-21.02,;27.62,-21.79,;27.62,-23.33,;26.29,-21.01,;26.29,-19.48,;27.62,-18.71,;24.96,-21.78,;23.62,-21.01,;23.63,-19.47,;22.28,-21.79,;22.29,-23.32,;20.95,-21.02,;19.62,-21.79,;19.62,-23.33,;18.29,-21.03,;18.28,-19.48,;19.62,-18.71,;19.61,-17.17,;20.94,-16.4,;20.94,-14.86,;19.61,-14.09,;22.27,-14.08,;16.96,-21.8,;15.62,-21.03,;15.62,-19.49,;14.29,-21.8,;14.29,-23.34,;15.63,-24.11,;17.04,-23.48,;18.07,-24.62,;17.29,-25.96,;17.78,-27.42,;16.75,-28.57,;15.24,-28.25,;14.76,-26.79,;15.79,-25.64,;12.95,-21.04,;11.62,-21.81,;11.62,-23.35,;10.29,-21.04,;10.28,-19.51,;11.61,-18.73,;11.61,-17.19,;12.94,-16.41,;12.94,-14.88,;11.61,-14.11,;14.27,-14.1,;8.95,-21.82,;7.62,-21.05,;7.62,-19.51,;6.29,-21.83,;6.29,-23.36,;7.62,-24.13,;7.63,-25.67,;8.96,-26.43,;8.97,-27.97,;7.64,-28.75,;10.3,-28.74,;4.95,-21.05,;3.62,-21.83,;3.62,-23.37,;2.28,-21.06,;.95,-21.84,;-.38,-21.07,;-.39,-19.53,;-1.71,-21.84,;-1.71,-23.38,;-.38,-24.15,;1.03,-23.52,;2.06,-24.66,;1.3,-26,;1.77,-27.45,;.75,-28.6,;-.76,-28.29,;-1.24,-26.82,;-.21,-25.68,;-3.05,-21.07,;-4.38,-21.84,;-4.38,-23.39,;-5.72,-21.08,;-7.05,-21.85,;-5.72,-19.54,;-4.39,-18.77,;-2.98,-19.39,;-1.95,-18.24,;-2.73,-16.91,;-2.25,-15.45,;-3.29,-14.3,;-4.79,-14.63,;-5.27,-16.09,;-4.23,-17.23,;2.28,-19.52,;.95,-18.76,;3.61,-18.75,;43.62,-21.81,;43.62,-23.34,;44.96,-21.04,;46.29,-21.81,;46.29,-23.35,;47.62,-21.04,;47.62,-19.5,;48.95,-21.81,;50.29,-21.04,;50.3,-19.5,;51.62,-18.73,;51.62,-17.2,;52.96,-16.42,;52.96,-14.88,;51.63,-14.1,;54.3,-14.11,;51.63,-21.81,;52.95,-21.04,;51.62,-23.36,)| Show InChI InChI=1S/C90H139N31O18/c1-46(2)36-66(76(128)108-44-71(124)111-67(37-47(3)4)81(133)110-48(5)73(125)116-65(86(138)139)30-19-35-104-90(99)100)118-79(131)61(26-14-15-31-91)114-84(136)70(45-122)120-74(126)49(6)109-77(129)62(27-16-32-101-87(93)94)113-82(134)68(39-52-42-106-59-24-12-9-21-55(52)59)119-80(132)63(28-17-33-102-88(95)96)112-78(130)64(29-18-34-103-89(97)98)115-85(137)72(50(7)123)121-83(135)69(40-53-43-107-60-25-13-10-22-56(53)60)117-75(127)57(92)38-51-41-105-58-23-11-8-20-54(51)58/h8-13,20-25,41-43,46-50,57,61-70,72,105-107,122-123H,14-19,26-40,44-45,91-92H2,1-7H3,(H,108,128)(H,109,129)(H,110,133)(H,111,124)(H,112,130)(H,113,134)(H,114,136)(H,115,137)(H,116,125)(H,117,127)(H,118,131)(H,119,132)(H,120,126)(H,121,135)(H,138,139)(H4,93,94,101)(H4,95,96,102)(H4,97,98,103)(H4,99,100,104)/t48-,49-,50+,57-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 81.3 | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Agonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant by degranulation assay |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50028695

(CHEMBL3342688)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1c[nH]c(n1)C(c1ccccc1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C23H26N6O3/c24-23(25)26-13-7-12-17(22(31)32)29-21(30)18-14-27-20(28-18)19(15-8-3-1-4-9-15)16-10-5-2-6-11-16/h1-6,8-11,14,17,19H,7,12-13H2,(H,27,28)(H,29,30)(H,31,32)(H4,24,25,26)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity against C3AR in HMDMs assessed as increase in intracellular calcium release by Fluo-3 AM dye based FLIPR assay relative to 1 uM C3a |
J Med Chem 57: 8459-70 (2014)
Article DOI: 10.1021/jm500956p
BindingDB Entry DOI: 10.7270/Q21R6S3N |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50587343
(CHEMBL5080181)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 129 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human C3aR expressed in CHO cells assessed as induction of ERK1/2 phosphorylation at Thr202/Tyr204 residues incubated for 10 mins... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01174
BindingDB Entry DOI: 10.7270/Q2WS8Z5G |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50322618
((3S,6R,9S)-14-amino-2-((S)-1-((S)-2-((S)-1-((S)-6-...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6])-[#6@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C58H90N14O13S/c1-34(2)30-44(54(81)70(4)35(3)48(75)66-42(57(84)85)17-11-26-63-58(61)62)68-53(80)47-19-13-28-72(47)56(83)41(24-29-86-5)65-52(79)46-18-12-27-71(46)55(82)40(16-9-10-25-59)64-50(77)43(32-36-14-7-6-8-15-36)67-51(78)45(33-73)69-49(76)39(60)31-37-20-22-38(74)23-21-37/h6-8,14-15,20-23,34-35,39-47,73-74H,9-13,16-19,24-33,59-60H2,1-5H3,(H,64,77)(H,65,79)(H,66,75)(H,67,78)(H,68,80)(H,69,76)(H,84,85)(H4,61,62,63)/t35-,39+,40+,41+,42+,43+,44+,45+,46+,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at human C3a receptor in human U937 cells assessed as induction of intracellular calcium release |
J Med Chem 53: 4938-48 (2010)
Article DOI: 10.1021/jm1003705
BindingDB Entry DOI: 10.7270/Q2QR4X9F |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50028695

(CHEMBL3342688)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1c[nH]c(n1)C(c1ccccc1)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C23H26N6O3/c24-23(25)26-13-7-12-17(22(31)32)29-21(30)18-14-27-20(28-18)19(15-8-3-1-4-9-15)16-10-5-2-6-11-16/h1-6,8-11,14,17,19H,7,12-13H2,(H,27,28)(H,29,30)(H,31,32)(H4,24,25,26)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity against C3AR in HMDMs assessed as increase in intracellular calcium release by Fluo-3 AM dye based FLIPR assay relative to 1 uM C3a |
J Med Chem 57: 8459-70 (2014)
Article DOI: 10.1021/jm500956p
BindingDB Entry DOI: 10.7270/Q21R6S3N |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50322618

((3S,6R,9S)-14-amino-2-((S)-1-((S)-2-((S)-1-((S)-6-...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7](-[#6])-[#6@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C58H90N14O13S/c1-34(2)30-44(54(81)70(4)35(3)48(75)66-42(57(84)85)17-11-26-63-58(61)62)68-53(80)47-19-13-28-72(47)56(83)41(24-29-86-5)65-52(79)46-18-12-27-71(46)55(82)40(16-9-10-25-59)64-50(77)43(32-36-14-7-6-8-15-36)67-51(78)45(33-73)69-49(76)39(60)31-37-20-22-38(74)23-21-37/h6-8,14-15,20-23,34-35,39-47,73-74H,9-13,16-19,24-33,59-60H2,1-5H3,(H,64,77)(H,65,79)(H,66,75)(H,67,78)(H,68,80)(H,69,76)(H,84,85)(H4,61,62,63)/t35-,39+,40+,41+,42+,43+,44+,45+,46+,47+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Antagonist activity at human C3a receptor in human U937 cells assessed as inhibition of intracellular calcium mobilization |
J Med Chem 53: 4938-48 (2010)
Article DOI: 10.1021/jm1003705
BindingDB Entry DOI: 10.7270/Q2QR4X9F |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50389006
(CHEMBL2064020)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:107.123,87.91,65.74,40.47,29.31,12.16,4.4,130.135,wD:93.107,76.85,51.63,35.35,20.25,125.131,(42.57,1.47,;42.59,-.07,;43.93,-.82,;41.26,-.85,;41.27,-2.4,;39.95,-3.19,;38.61,-2.41,;38.59,-.89,;37.29,-3.2,;35.95,-2.45,;34.62,-3.23,;34.63,-4.77,;33.27,-2.48,;33.26,-.94,;34.59,-.15,;34.57,1.38,;35.93,-.9,;31.95,-3.26,;30.61,-2.5,;30.59,-.97,;29.28,-3.28,;29.3,-4.82,;30.64,-5.58,;30.66,-7.12,;32,-7.88,;32.01,-9.42,;27.94,-2.53,;26.62,-3.32,;26.63,-4.85,;25.28,-2.55,;25.26,-1.02,;26.58,-.24,;23.95,-3.34,;22.6,-2.58,;22.6,-1.05,;21.28,-3.37,;21.3,-4.91,;19.94,-2.61,;18.62,-3.41,;18.63,-4.94,;17.28,-2.64,;17.26,-1.11,;18.58,-.32,;18.57,1.21,;19.89,2,;19.87,3.54,;18.53,4.29,;21.2,4.32,;15.96,-3.43,;14.61,-2.68,;14.6,-1.14,;13.28,-3.46,;13.3,-5,;14.64,-5.75,;16.04,-5.11,;17.08,-6.24,;16.33,-7.58,;16.82,-9.04,;15.8,-10.2,;14.29,-9.9,;13.8,-8.44,;14.82,-7.28,;11.94,-2.7,;10.62,-3.49,;10.64,-5.03,;9.28,-2.73,;9.26,-1.19,;10.58,-.41,;10.57,1.13,;11.89,1.92,;11.87,3.45,;10.53,4.2,;13.2,4.23,;7.95,-3.51,;6.61,-2.76,;6.59,-1.22,;5.28,-3.54,;5.3,-5.08,;6.64,-5.84,;6.66,-7.38,;8,-8.13,;8.01,-9.67,;6.69,-10.45,;9.36,-10.43,;3.94,-2.79,;2.61,-3.57,;2.63,-5.11,;1.27,-2.82,;1.26,-1.27,;2.58,-.49,;-.05,-3.6,;-1.39,-2.84,;-1.41,-1.3,;-2.72,-3.63,;-2.7,-5.17,;-1.36,-5.92,;.04,-5.28,;1.08,-6.42,;.32,-7.76,;.82,-9.22,;-.2,-10.37,;-1.71,-10.07,;-2.2,-8.61,;-1.19,-7.45,;-4.06,-2.87,;-5.39,-3.66,;-5.37,-5.2,;-6.73,-2.9,;-6.74,-1.36,;-5.42,-.57,;-4.01,-1.18,;-2.99,-.03,;-3.77,1.3,;-3.31,2.77,;-4.36,3.9,;-5.85,3.56,;-6.32,2.09,;-5.27,.96,;-8.05,-3.68,;-9.38,-2.92,;-10.71,-3.69,;-9.39,-1.39,;42.61,-3.15,;42.63,-4.69,;43.94,-2.37,;45.29,-3.12,;45.3,-4.66,;46.61,-2.34,;46.59,-.8,;47.95,-3.09,;49.28,-2.31,;49.27,-.77,;50.59,.02,;50.57,1.55,;51.89,2.35,;51.88,3.88,;50.53,4.63,;53.21,4.66,;50.62,-3.07,;51.94,-2.28,;50.63,-4.61,)| Show InChI InChI=1S/C91H139N31O19/c1-47(2)36-66(76(129)108-44-73(126)112-67(37-48(3)4)81(134)110-49(5)74(127)117-65(87(140)141)30-19-35-104-91(99)100)118-79(132)61(26-14-15-31-92)115-85(138)71(45-123)121-75(128)50(6)109-77(130)62(27-16-32-101-88(93)94)114-83(136)69(39-53-42-106-59-24-12-9-21-56(53)59)119-80(133)64(29-18-34-103-90(97)98)113-78(131)63(28-17-33-102-89(95)96)116-86(139)72(46-124)122-84(137)70(40-54-43-107-60-25-13-10-22-57(54)60)120-82(135)68(111-51(7)125)38-52-41-105-58-23-11-8-20-55(52)58/h8-13,20-25,41-43,47-50,61-72,105-107,123-124H,14-19,26-40,44-46,92H2,1-7H3,(H,108,129)(H,109,130)(H,110,134)(H,111,125)(H,112,126)(H,113,131)(H,114,136)(H,115,138)(H,116,139)(H,117,127)(H,118,132)(H,119,133)(H,120,135)(H,121,128)(H,122,137)(H,140,141)(H4,93,94,101)(H4,95,96,102)(H4,97,98,103)(H4,99,100,104)/t49-,50-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 148 | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Agonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant by degranulation assay |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50388998
(CHEMBL2064012)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)NC(=O)CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:99.114,63.69,40.47,29.31,12.16,4.4,122.126,wD:85.98,72.78,51.60,35.35,20.25,117.122,(47,-28.76,;47,-30.3,;48.34,-31.08,;45.67,-31.07,;45.66,-32.61,;44.33,-33.38,;43.01,-32.61,;43,-31.07,;41.67,-33.37,;40.33,-32.61,;39,-33.38,;39,-34.91,;37.66,-32.6,;37.67,-31.07,;39,-30.29,;39.01,-28.75,;40.34,-31.07,;36.34,-33.37,;35,-32.6,;35,-31.06,;33.66,-33.36,;33.66,-34.91,;34.99,-35.68,;34.99,-37.22,;36.33,-37.99,;36.32,-39.53,;32.34,-32.6,;31,-33.37,;31,-34.91,;29.67,-32.6,;29.67,-31.06,;31.01,-30.28,;28.33,-33.37,;27,-32.59,;26.99,-31.06,;25.66,-33.36,;25.66,-34.9,;24.33,-32.6,;22.99,-33.36,;22.99,-34.9,;21.67,-32.59,;21.67,-31.06,;23,-30.28,;23.01,-28.74,;24.33,-27.97,;24.33,-26.44,;23.01,-25.66,;25.67,-25.66,;20.33,-33.36,;19,-32.58,;18.99,-31.05,;17.66,-33.35,;17.66,-34.89,;19,-35.67,;20.33,-34.89,;21.66,-35.67,;21.67,-37.21,;22.99,-37.98,;20.33,-37.97,;18.99,-37.21,;16.33,-32.58,;14.99,-33.35,;14.99,-34.9,;13.66,-32.59,;13.66,-31.04,;15,-30.27,;15,-28.74,;16.33,-27.97,;16.33,-26.42,;12.33,-33.35,;10.99,-32.58,;10.99,-31.04,;9.66,-33.35,;9.66,-34.88,;11,-35.66,;10.99,-37.2,;12.33,-37.97,;12.33,-39.51,;8.33,-32.58,;6.99,-33.34,;6.99,-34.88,;5.66,-32.58,;4.32,-33.34,;2.99,-32.57,;2.99,-31.03,;1.67,-33.34,;1.66,-34.88,;2.99,-35.65,;4.39,-35.03,;5.43,-36.17,;4.66,-37.5,;5.13,-38.97,;4.1,-40.12,;2.59,-39.8,;2.12,-38.33,;3.16,-37.19,;.33,-32.57,;-1.01,-33.34,;-1.01,-34.87,;-2.34,-32.57,;-2.34,-31.03,;-1.01,-30.26,;.4,-30.89,;1.43,-29.75,;.66,-28.41,;1.14,-26.94,;.11,-25.8,;-1.39,-26.12,;-1.88,-27.58,;-.84,-28.73,;-3.68,-33.33,;-5.01,-32.57,;-6.33,-33.34,;-5.01,-31.04,;47,-33.38,;46.99,-34.92,;48.33,-32.61,;49.67,-33.38,;49.67,-34.92,;51,-32.62,;51.01,-31.07,;52.34,-33.38,;53.67,-32.62,;53.67,-31.08,;55,-30.31,;55,-28.77,;56.34,-28,;56.34,-26.46,;55.01,-25.69,;57.67,-25.69,;55,-33.39,;56.33,-32.62,;55,-34.92,)| Show InChI InChI=1S/C88H136N26O19/c1-48(2)38-66(76(122)100-46-73(119)106-67(39-49(3)4)82(128)103-50(5)74(120)110-65(86(132)133)28-19-37-97-88(94)95)111-80(126)63(26-14-17-35-91)109-85(131)71(47-115)114-75(121)51(6)102-78(124)64(27-18-36-96-87(92)93)108-83(129)68(40-53-29-31-56(117)32-30-53)112-81(127)62(25-13-16-34-90)107-79(125)61(24-12-15-33-89)105-72(118)45-101-77(123)69(41-54-43-98-59-22-10-8-20-57(54)59)113-84(130)70(104-52(7)116)42-55-44-99-60-23-11-9-21-58(55)60/h8-11,20-23,29-32,43-44,48-51,61-71,98-99,115,117H,12-19,24-28,33-42,45-47,89-91H2,1-7H3,(H,100,122)(H,101,123)(H,102,124)(H,103,128)(H,104,116)(H,105,118)(H,106,119)(H,107,125)(H,108,129)(H,109,131)(H,110,120)(H,111,126)(H,112,127)(H,113,130)(H,114,121)(H,132,133)(H4,92,93,96)(H4,94,95,97)/t50-,51-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Agonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant by degranulation assay |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50388996
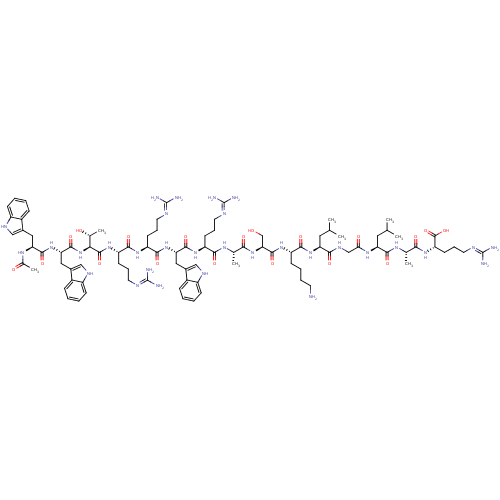
(CHEMBL2063898)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:105.121,87.89,120.127,65.74,40.47,29.31,12.16,4.4,131.136,wD:91.105,76.85,51.63,35.35,20.25,126.132,(45.71,2.34,;45.71,.8,;47.04,.03,;44.38,.03,;44.37,-1.5,;43.04,-2.28,;41.7,-1.5,;41.71,.04,;40.37,-2.27,;39.04,-1.5,;37.71,-2.27,;37.7,-3.81,;36.37,-1.5,;36.38,.04,;37.71,.81,;37.71,2.35,;39.04,.04,;35.04,-2.27,;33.7,-1.49,;33.7,.04,;32.37,-2.26,;32.37,-3.8,;33.7,-4.57,;33.7,-6.12,;35.03,-6.89,;35.03,-8.43,;31.04,-1.49,;29.7,-2.26,;29.7,-3.8,;28.37,-1.49,;28.37,.05,;29.71,.82,;27.04,-2.26,;25.7,-1.49,;25.71,.06,;24.37,-2.26,;24.37,-3.8,;23.04,-1.49,;21.71,-2.26,;21.71,-3.81,;20.37,-1.5,;20.37,.04,;21.7,.82,;21.7,2.35,;23.03,3.13,;23.02,4.67,;21.69,5.43,;24.36,5.45,;19.04,-2.27,;17.71,-1.5,;17.7,.03,;16.38,-2.28,;16.37,-3.81,;17.71,-4.58,;19.12,-3.95,;20.15,-5.09,;19.38,-6.43,;19.86,-7.89,;18.84,-9.04,;17.32,-8.72,;16.85,-7.26,;17.87,-6.11,;15.03,-1.51,;13.7,-2.28,;13.71,-3.82,;12.37,-1.51,;12.37,.02,;13.7,.8,;13.69,2.34,;15.03,3.11,;15.02,4.65,;13.69,5.42,;16.35,5.42,;11.04,-2.29,;9.7,-1.52,;9.7,.02,;8.37,-2.3,;8.37,-3.83,;9.71,-4.6,;9.72,-6.14,;11.05,-6.9,;11.05,-8.45,;9.72,-9.22,;12.38,-9.21,;7.03,-1.53,;5.7,-2.3,;5.71,-3.84,;4.37,-1.54,;3.04,-2.31,;1.7,-1.54,;1.7,-0,;.37,-2.31,;.38,-3.85,;1.71,-4.62,;3.12,-3.99,;4.15,-5.13,;3.38,-6.47,;3.86,-7.93,;2.83,-9.07,;1.33,-8.76,;.84,-7.3,;1.88,-6.15,;-.97,-1.55,;-2.3,-2.32,;-2.3,-3.86,;-3.63,-1.55,;-3.64,-.02,;-2.31,.76,;-.9,.14,;.13,1.29,;-.64,2.61,;-.17,4.08,;-1.2,5.22,;-2.71,4.9,;-3.18,3.44,;-2.15,2.3,;-4.97,-2.33,;-6.3,-1.56,;-7.63,-2.34,;-6.31,-.02,;4.36,.01,;3.03,.77,;5.7,.78,;45.71,-2.28,;45.71,-3.82,;47.04,-1.51,;48.37,-2.28,;48.37,-3.82,;49.71,-1.51,;49.71,.03,;51.04,-2.29,;52.37,-1.52,;52.38,.02,;53.71,.8,;53.71,2.33,;55.04,3.1,;55.05,4.64,;53.72,5.43,;56.38,5.42,;53.71,-2.29,;55.04,-1.51,;53.7,-3.83,)| Show InChI InChI=1S/C92H141N31O19/c1-47(2)37-67(77(130)109-45-73(127)113-68(38-48(3)4)82(135)111-49(5)75(128)118-66(88(141)142)31-20-36-105-92(100)101)119-80(133)62(27-15-16-32-93)116-86(139)72(46-124)122-76(129)50(6)110-78(131)63(28-17-33-102-89(94)95)115-84(137)70(40-54-43-107-60-25-13-10-22-57(54)60)120-81(134)64(29-18-34-103-90(96)97)114-79(132)65(30-19-35-104-91(98)99)117-87(140)74(51(7)125)123-85(138)71(41-55-44-108-61-26-14-11-23-58(55)61)121-83(136)69(112-52(8)126)39-53-42-106-59-24-12-9-21-56(53)59/h9-14,21-26,42-44,47-51,62-72,74,106-108,124-125H,15-20,27-41,45-46,93H2,1-8H3,(H,109,130)(H,110,131)(H,111,135)(H,112,126)(H,113,127)(H,114,132)(H,115,137)(H,116,139)(H,117,140)(H,118,128)(H,119,133)(H,120,134)(H,121,136)(H,122,129)(H,123,138)(H,141,142)(H4,94,95,102)(H4,96,97,103)(H4,98,99,104)(H4,100,101,105)/t49-,50-,51+,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,74-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 195 | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Agonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant by degranulation assay |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50322620

((S)-2-((R)-2-((S)-2-((S)-1-((S)-2-((S)-1-((S)-6-am...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C57H88N14O13S/c1-33(2)29-42(49(76)63-34(3)47(74)66-41(56(83)84)16-10-25-62-57(60)61)68-53(80)46-18-12-27-71(46)55(82)40(23-28-85-4)65-52(79)45-17-11-26-70(45)54(81)39(15-8-9-24-58)64-50(77)43(31-35-13-6-5-7-14-35)67-51(78)44(32-72)69-48(75)38(59)30-36-19-21-37(73)22-20-36/h5-7,13-14,19-22,33-34,38-46,72-73H,8-12,15-18,23-32,58-59H2,1-4H3,(H,63,76)(H,64,77)(H,65,79)(H,66,74)(H,67,78)(H,68,80)(H,69,75)(H,83,84)(H4,60,61,62)/t34-,38+,39+,40+,41+,42+,43+,44+,45+,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at human C3a receptor in human U937 cells assessed as induction of intracellular calcium release |
J Med Chem 53: 4938-48 (2010)
Article DOI: 10.1021/jm1003705
BindingDB Entry DOI: 10.7270/Q2QR4X9F |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
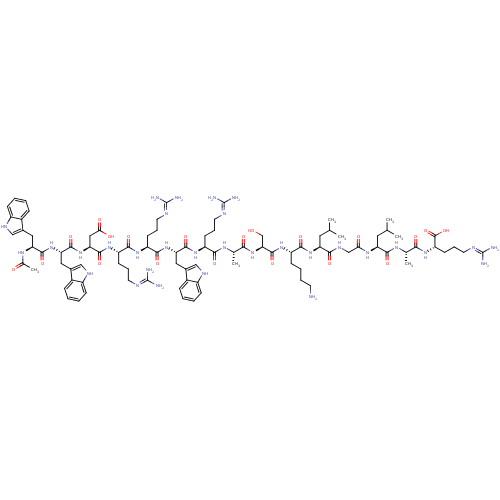
(Homo sapiens (Human)) | BDBM50389003
(CHEMBL2064018)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O |r,wU:109.125,87.93,65.74,40.47,29.31,12.16,4.4,132.137,wD:95.109,76.85,51.63,35.35,20.25,127.133,(46.4,-15.18,;46.39,-16.72,;47.73,-17.49,;45.05,-17.49,;45.05,-19.03,;43.72,-19.8,;42.38,-19.02,;42.39,-17.48,;41.05,-19.8,;39.72,-19.02,;38.38,-19.78,;38.38,-21.32,;37.05,-19.01,;37.05,-17.48,;38.39,-16.7,;38.39,-15.16,;39.72,-17.48,;35.71,-19.78,;34.38,-19.01,;34.38,-17.47,;33.04,-19.77,;33.04,-21.31,;34.37,-22.09,;34.37,-23.63,;35.7,-24.4,;35.7,-25.94,;31.71,-19,;30.38,-19.77,;30.37,-21.31,;29.04,-18.99,;29.05,-17.46,;30.38,-16.69,;27.7,-19.77,;26.37,-18.99,;26.38,-17.45,;25.04,-19.76,;25.03,-21.3,;23.71,-18.99,;22.37,-19.75,;22.37,-21.29,;21.04,-18.98,;21.04,-17.44,;22.38,-16.67,;22.38,-15.13,;23.71,-14.37,;23.72,-12.82,;22.38,-12.05,;25.06,-12.05,;19.71,-19.75,;18.38,-18.97,;18.38,-17.43,;17.03,-19.74,;17.03,-21.28,;18.36,-22.05,;19.77,-21.43,;20.8,-22.58,;20.03,-23.91,;20.5,-25.38,;19.47,-26.52,;17.96,-26.19,;17.49,-24.73,;18.52,-23.59,;15.7,-18.97,;14.37,-19.73,;14.37,-21.28,;13.04,-18.96,;13.04,-17.43,;14.37,-16.65,;14.38,-15.11,;15.71,-14.35,;15.71,-12.81,;14.38,-12.03,;17.05,-12.04,;11.7,-19.73,;10.36,-18.96,;10.37,-17.41,;9.03,-19.72,;9.03,-21.26,;10.36,-22.04,;10.36,-23.58,;11.69,-24.35,;11.69,-25.89,;10.35,-26.66,;13.02,-26.66,;7.7,-18.95,;6.36,-19.72,;6.36,-21.26,;5.03,-18.94,;5.03,-17.4,;6.37,-16.64,;7.7,-17.42,;6.37,-15.1,;3.7,-19.72,;2.36,-18.94,;2.37,-17.4,;1.04,-19.7,;1.03,-21.24,;2.37,-22.02,;3.77,-21.4,;4.79,-22.55,;4.02,-23.88,;4.49,-25.34,;3.46,-26.48,;1.97,-26.16,;1.47,-24.69,;2.52,-23.56,;-.3,-18.93,;-1.64,-19.7,;-1.64,-21.24,;-2.97,-18.93,;-2.96,-17.38,;-1.64,-16.62,;-.24,-17.25,;.8,-16.1,;.03,-14.77,;.52,-13.3,;-.51,-12.16,;-2.02,-12.48,;-2.5,-13.94,;-1.48,-15.08,;-4.3,-19.7,;-5.63,-18.93,;-6.96,-19.71,;-5.63,-17.4,;46.38,-19.8,;46.38,-21.34,;47.72,-19.04,;49.06,-19.81,;49.05,-21.35,;50.39,-19.04,;50.39,-17.5,;51.72,-19.81,;53.06,-19.05,;53.06,-17.51,;54.4,-16.74,;54.4,-15.2,;55.73,-14.43,;55.74,-12.89,;54.4,-12.12,;57.08,-12.13,;54.39,-19.82,;55.73,-19.05,;54.38,-21.36,)| Show InChI InChI=1S/C92H139N31O20/c1-47(2)36-66(77(131)109-45-73(126)113-67(37-48(3)4)82(136)111-49(5)75(129)118-65(88(142)143)30-19-35-105-92(100)101)119-80(134)61(26-14-15-31-93)117-87(141)72(46-124)123-76(130)50(6)110-78(132)62(27-16-32-102-89(94)95)115-84(138)69(39-53-43-107-59-24-12-9-21-56(53)59)120-81(135)64(29-18-34-104-91(98)99)114-79(133)63(28-17-33-103-90(96)97)116-86(140)71(41-74(127)128)122-85(139)70(40-54-44-108-60-25-13-10-22-57(54)60)121-83(137)68(112-51(7)125)38-52-42-106-58-23-11-8-20-55(52)58/h8-13,20-25,42-44,47-50,61-72,106-108,124H,14-19,26-41,45-46,93H2,1-7H3,(H,109,131)(H,110,132)(H,111,136)(H,112,125)(H,113,126)(H,114,133)(H,115,138)(H,116,140)(H,117,141)(H,118,129)(H,119,134)(H,120,135)(H,121,137)(H,122,139)(H,123,130)(H,127,128)(H,142,143)(H4,94,95,102)(H4,96,97,103)(H4,98,99,104)(H4,100,101,105)/t49-,50-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 224 | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Agonist activity at human C3a receptor expressed in RBL-2H3 cells assessed as beta-hexosaminidase activity in cell supernatant by degranulation assay |
J Med Chem 55: 4159-68 (2012)
Article DOI: 10.1021/jm201609k
BindingDB Entry DOI: 10.7270/Q24F1RS4 |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499182

(CHEMBL3736020)Show SMILES CC[C@H](C)[C@H](NC(=O)C1CCCc2ccccc12)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H36N6O5/c1-3-15(2)21(32-22(33)18-11-6-9-16-8-4-5-10-17(16)18)24-31-20(14-37-24)23(34)30-19(25(35)36)12-7-13-29-26(27)28/h4-5,8,10,14-15,18-19,21H,3,6-7,9,11-13H2,1-2H3,(H,30,34)(H,32,33)(H,35,36)(H4,27,28,29)/t15-,18?,19-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
(Homo sapiens (Human)) | BDBM50499182

(CHEMBL3736020)Show SMILES CC[C@H](C)[C@H](NC(=O)C1CCCc2ccccc12)c1nc(co1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H36N6O5/c1-3-15(2)21(32-22(33)18-11-6-9-16-8-4-5-10-17(16)18)24-31-20(14-37-24)23(34)30-19(25(35)36)12-7-13-29-26(27)28/h4-5,8,10,14-15,18-19,21H,3,6-7,9,11-13H2,1-2H3,(H,30,34)(H,32,33)(H,35,36)(H4,27,28,29)/t15-,18?,19-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at C3aR in human MDM cells assessed as inhibition of C3a-induced Ca2+ response measured for 60 secs by fluorescence assay |
Bioorg Med Chem Lett 25: 5604-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.038
BindingDB Entry DOI: 10.7270/Q2Q2437Q |
More data for this
Ligand-Target Pair | |
C3a anaphylatoxin chemotactic receptor
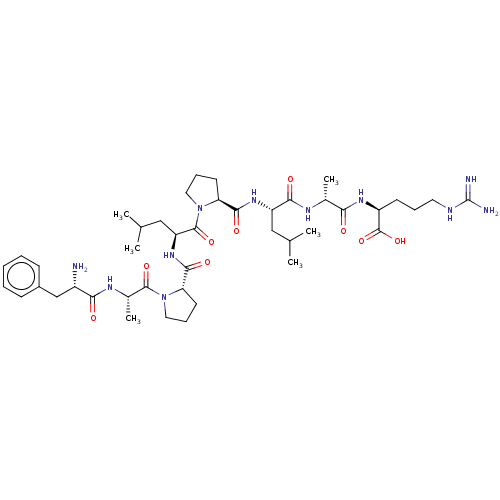
(Homo sapiens (Human)) | BDBM50587345
(CHEMBL5092449)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 282 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human C3aR expressed in CHO cells assessed as induction of ERK1/2 phosphorylation at Thr202/Tyr204 residues incubated for 10 mins... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01174
BindingDB Entry DOI: 10.7270/Q2WS8Z5G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































