 Found 335 hits of ki for UniProtKB: Q15661
Found 335 hits of ki for UniProtKB: Q15661 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083552

(1,9-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C37H44N8O6/c38-34(39)24-8-10-28-26(20-24)22-30(50-28)36(48)44-16-12-42(13-17-44)32(46)6-4-2-1-3-5-7-33(47)43-14-18-45(19-15-43)37(49)31-23-27-21-25(35(40)41)9-11-29(27)51-31/h8-11,20-23H,1-7,12-19H2,(H3,38,39)(H3,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083561

(1-{4-[5-amino(imino)methylbenzo[b]thiophen-2-ylcar...)Show SMILES NC(=N)c1ccc2sc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3s2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O6S2/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083556
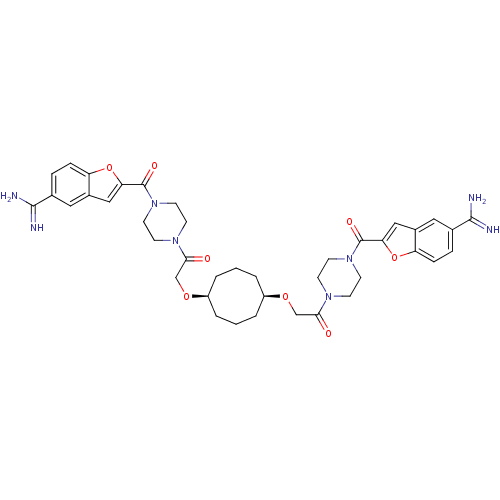
(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CO[C@H]1CCC[C@H](CCC1)OCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C40H48N8O8/c41-37(42)25-7-9-31-27(19-25)21-33(55-31)39(51)47-15-11-45(12-16-47)35(49)23-53-29-3-1-4-30(6-2-5-29)54-24-36(50)46-13-17-48(18-14-46)40(52)34-22-28-20-26(38(43)44)8-10-32(28)56-34/h7-10,19-22,29-30H,1-6,11-18,23-24H2,(H3,41,42)(H3,43,44)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083541
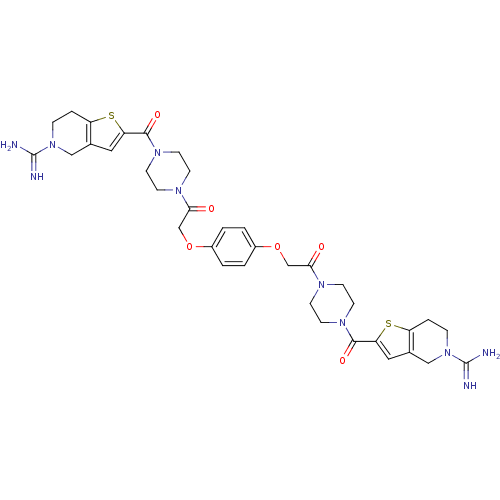
(1-{4-[5-amino(imino)methyl-4,5,6,7-tetrahydrothien...)Show SMILES NC(=N)N1CCc2sc(cc2C1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3CN(CCc3s2)C(N)=N)cc1 Show InChI InChI=1S/C36H44N10O6S2/c37-35(38)45-7-5-27-23(19-45)17-29(53-27)33(49)43-13-9-41(10-14-43)31(47)21-51-25-1-2-26(4-3-25)52-22-32(48)42-11-15-44(16-12-42)34(50)30-18-24-20-46(36(39)40)8-6-28(24)54-30/h1-4,17-18H,5-16,19-22H2,(H3,37,38)(H3,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083548

(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccc(OCC(=O)N2CCN(CC2)C(=O)c2cc3cc(ccc3o2)C(N)=N)cc1 Show InChI InChI=1S/C38H38N8O8/c39-35(40)23-1-7-29-25(17-23)19-31(53-29)37(49)45-13-9-43(10-14-45)33(47)21-51-27-3-5-28(6-4-27)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)2-8-30(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093142
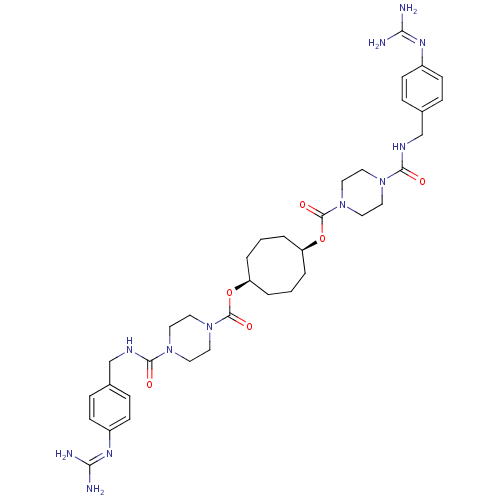
(1,5-di{4-[4-amino(imino)methylaminobenzylcarbamoyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C36H52N12O6/c37-31(38)43-27-11-7-25(8-12-27)23-41-33(49)45-15-19-47(20-16-45)35(51)53-29-3-1-4-30(6-2-5-29)54-36(52)48-21-17-46(18-22-48)34(50)42-24-26-9-13-28(14-10-26)44-32(39)40/h7-14,29-30H,1-6,15-24H2,(H,41,49)(H,42,50)(H4,37,38,43)(H4,39,40,44)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase enzyme |
Bioorg Med Chem Lett 11: 2325-30 (2001)
BindingDB Entry DOI: 10.7270/Q21835S6 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50218667

(APC-1390 | CHEMBL46809)Show SMILES NC(=N)Nc1ccc(CNC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)NCc2ccc(NC(N)=N)cc2)cc1 Show InChI InChI=1S/C36H52N12O6/c37-31(38)43-27-11-7-25(8-12-27)23-41-33(49)45-15-19-47(20-16-45)35(51)53-29-3-1-4-30(6-2-5-29)54-36(52)48-21-17-46(18-22-48)34(50)42-24-26-9-13-28(14-10-26)44-32(39)40/h7-14,29-30H,1-6,15-24H2,(H,41,49)(H,42,50)(H4,37,38,43)(H4,39,40,44)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT3 serotonin receptor in NG 108-15 neuroblastoma glioma cells using [3H]-GR-65,630 radioligand. |
Bioorg Med Chem Lett 11: 1629-33 (2001)
BindingDB Entry DOI: 10.7270/Q2R78DHK |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093175

(CHEMBL311655 | Derivative of APC-2059)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C34H50N12O4/c35-31(36)41-27-11-7-25(8-12-27)23-39-33(49)45-19-15-43(16-20-45)29(47)5-3-1-2-4-6-30(48)44-17-21-46(22-18-44)34(50)40-24-26-9-13-28(14-10-26)42-32(37)38/h7-14H,1-6,15-24H2,(H,39,49)(H,40,50)(H4,35,36,41)(H4,37,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093157

(CHEMBL431969 | Derivative of piperazine-1-carboxyl...)Show SMILES NCc1ccc(CNC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)NCc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C36H52N8O6/c37-23-27-7-11-29(12-8-27)25-39-33(45)41-15-19-43(20-16-41)35(47)49-31-3-1-4-32(6-2-5-31)50-36(48)44-21-17-42(18-22-44)34(46)40-26-30-13-9-28(24-38)10-14-30/h7-14,31-32H,1-6,15-26,37-38H2,(H,39,45)(H,40,46)/t31-,32+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217306

(CHEMBL112049)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)CCCCCCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C36H42N8O6/c37-33(38)23-7-9-27-25(19-23)21-29(49-27)35(47)43-15-11-41(12-16-43)31(45)5-3-1-2-4-6-32(46)42-13-17-44(18-14-42)36(48)30-22-26-20-24(34(39)40)8-10-28(26)50-30/h7-10,19-22H,1-6,11-18H2,(H3,37,38)(H3,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093192
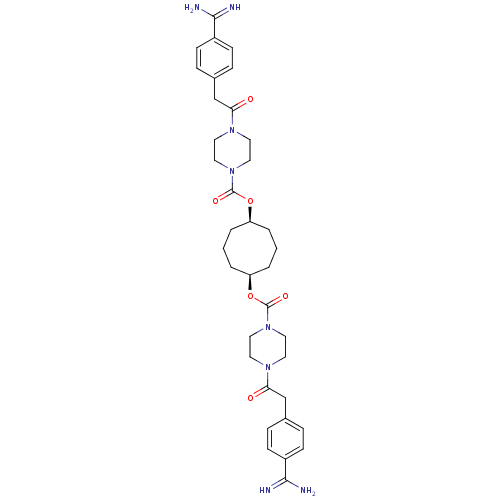
(CHEMBL311482 | Derivative of piperazine-1-carboxyl...)Show SMILES NC(=N)c1ccc(CC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)Cc2ccc(cc2)C(N)=N)cc1 Show InChI InChI=1S/C36H48N8O6/c37-33(38)27-11-7-25(8-12-27)23-31(45)41-15-19-43(20-16-41)35(47)49-29-3-1-4-30(6-2-5-29)50-36(48)44-21-17-42(18-22-44)32(46)24-26-9-13-28(14-10-26)34(39)40/h7-14,29-30H,1-6,15-24H2,(H3,37,38)(H3,39,40)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50101018
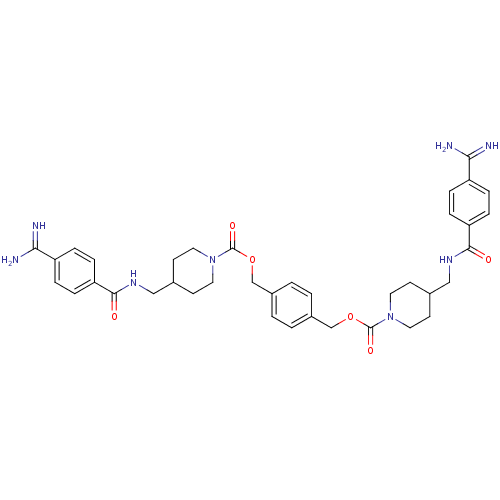
(1,4-di{4-[4-amino(imino)methylphenylcarboxamidomet...)Show SMILES NC(=N)c1ccc(cc1)C(=O)NCC1CCN(CC1)C(=O)OCc1ccc(COC(=O)N2CCC(CNC(=O)c3ccc(cc3)C(N)=N)CC2)cc1 Show InChI InChI=1S/C38H46N8O6/c39-33(40)29-5-9-31(10-6-29)35(47)43-21-25-13-17-45(18-14-25)37(49)51-23-27-1-2-28(4-3-27)24-52-38(50)46-19-15-26(16-20-46)22-44-36(48)32-11-7-30(8-12-32)34(41)42/h1-12,25-26H,13-24H2,(H3,39,40)(H3,41,42)(H,43,47)(H,44,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tryptase enzyme |
Bioorg Med Chem Lett 11: 2325-30 (2001)
BindingDB Entry DOI: 10.7270/Q21835S6 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50101018

(1,4-di{4-[4-amino(imino)methylphenylcarboxamidomet...)Show SMILES NC(=N)c1ccc(cc1)C(=O)NCC1CCN(CC1)C(=O)OCc1ccc(COC(=O)N2CCC(CNC(=O)c3ccc(cc3)C(N)=N)CC2)cc1 Show InChI InChI=1S/C38H46N8O6/c39-33(40)29-5-9-31(10-6-29)35(47)43-21-25-13-17-45(18-14-25)37(49)51-23-27-1-2-28(4-3-27)24-52-38(50)46-19-15-26(16-20-46)22-44-36(48)32-11-7-30(8-12-32)34(41)42/h1-12,25-26H,13-24H2,(H3,39,40)(H3,41,42)(H,43,47)(H,44,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT3 serotonin receptor in NG 108-15 neuroblastoma glioma cells using [3H]-GR-65,630 radioligand. |
Bioorg Med Chem Lett 11: 1629-33 (2001)
BindingDB Entry DOI: 10.7270/Q2R78DHK |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093167
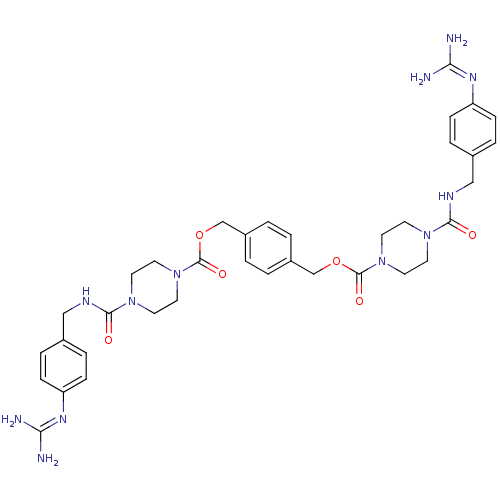
(CHEMBL75750 | Derivative of APC-2059)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-3-[#6]-[#6]-[#7](-[#6]-[#6]-3)-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C36H46N12O6/c37-31(38)43-29-9-5-25(6-10-29)21-41-33(49)45-13-17-47(18-14-45)35(51)53-23-27-1-2-28(4-3-27)24-54-36(52)48-19-15-46(16-20-48)34(50)42-22-26-7-11-30(12-8-26)44-32(39)40/h1-12H,13-24H2,(H,41,49)(H,42,50)(H4,37,38,43)(H4,39,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093156

(CHEMBL432172 | Derivative of APC-2059)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6]C23[#6]-[#6]C([#6]-[#8]-[#6](=O)-[#7]-4-[#6]-[#6]-[#7](-[#6]-[#6]-4)-[#6](=O)-[#7]-[#6]-c4ccc(cc4)\[#7]=[#6](/[#7])-[#7])([#6]-[#6]2)[#6]-[#6]3)cc1 Show InChI InChI=1S/C38H54N12O6/c39-31(40)45-29-5-1-27(2-6-29)23-43-33(51)47-15-19-49(20-16-47)35(53)55-25-37-9-12-38(13-10-37,14-11-37)26-56-36(54)50-21-17-48(18-22-50)34(52)44-24-28-3-7-30(8-4-28)46-32(41)42/h1-8H,9-26H2,(H,43,51)(H,44,52)(H4,39,40,45)(H4,41,42,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093154
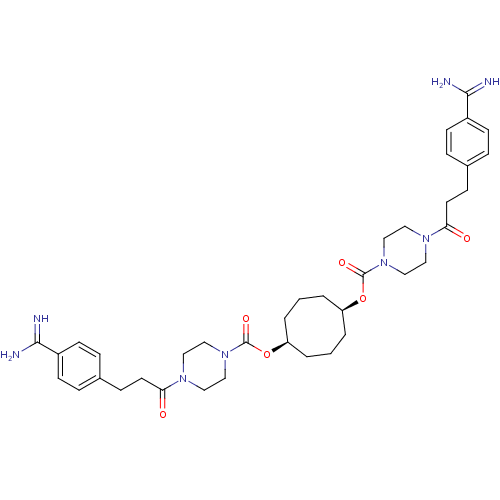
(CHEMBL448786 | Derivative of piperazine-1-carboxyl...)Show SMILES NC(=N)c1ccc(CCC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)CCc2ccc(cc2)C(N)=N)cc1 Show InChI InChI=1S/C38H52N8O6/c39-35(40)29-13-7-27(8-14-29)11-17-33(47)43-19-23-45(24-20-43)37(49)51-31-3-1-4-32(6-2-5-31)52-38(50)46-25-21-44(22-26-46)34(48)18-12-28-9-15-30(16-10-28)36(41)42/h7-10,13-16,31-32H,1-6,11-12,17-26H2,(H3,39,40)(H3,41,42)/t31-,32+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50218668

(CHEMBL42900)Show SMILES NCCCCCNC(=O)N1CCN(CC1)C(=O)O[C@H]1CCC[C@H](CCC1)OC(=O)N1CCN(CC1)C(=O)NCc1ccc(NC(N)=N)cc1 Show InChI InChI=1S/C33H54N10O6/c34-14-2-1-3-15-37-30(44)40-16-20-42(21-17-40)32(46)48-27-6-4-8-28(9-5-7-27)49-33(47)43-22-18-41(19-23-43)31(45)38-24-25-10-12-26(13-11-25)39-29(35)36/h10-13,27-28H,1-9,14-24,34H2,(H,37,44)(H,38,45)(H4,35,36,39)/t27-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT3 serotonin receptor in NG 108-15 neuroblastoma glioma cells using [3H]-GR-65,630 radioligand. |
Bioorg Med Chem Lett 11: 1629-33 (2001)
BindingDB Entry DOI: 10.7270/Q2R78DHK |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093173
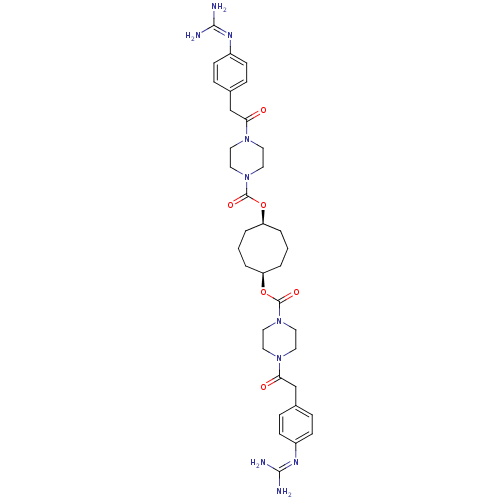
(CHEMBL309830 | Derivative of piperazine-1-carboxyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C36H50N10O6/c37-33(38)41-27-11-7-25(8-12-27)23-31(47)43-15-19-45(20-16-43)35(49)51-29-3-1-4-30(6-2-5-29)52-36(50)46-21-17-44(18-22-46)32(48)24-26-9-13-28(14-10-26)42-34(39)40/h7-14,29-30H,1-6,15-24H2,(H4,37,38,41)(H4,39,40,42)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093158

(1-{4-[4-amino(imino)methylaminobenzylcarbamoyl]hex...)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#8]-[#6@H]-1-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-1)-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)\[#7]=[#6](/[#7])-[#7] Show InChI InChI=1S/C33H54N10O6/c34-14-2-1-3-15-37-30(44)40-16-20-42(21-17-40)32(46)48-27-6-4-8-28(9-5-7-27)49-33(47)43-22-18-41(19-23-43)31(45)38-24-25-10-12-26(13-11-25)39-29(35)36/h10-13,27-28H,1-9,14-24,34H2,(H,37,44)(H,38,45)(H4,35,36,39)/t27-,28+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093199
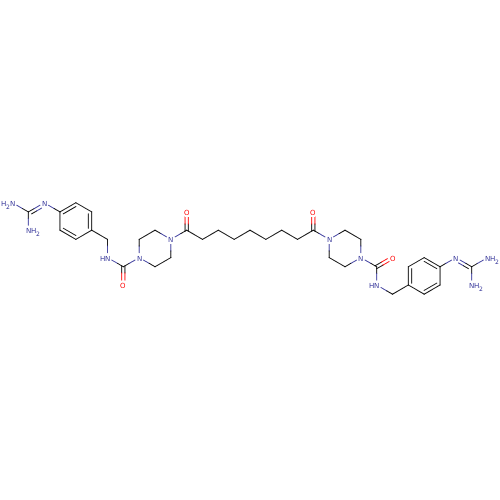
(CHEMBL75972 | Derivative of APC-2059)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C35H52N12O4/c36-32(37)42-28-12-8-26(9-13-28)24-40-34(50)46-20-16-44(17-21-46)30(48)6-4-2-1-3-5-7-31(49)45-18-22-47(23-19-45)35(51)41-25-27-10-14-29(15-11-27)43-33(38)39/h8-15H,1-7,16-25H2,(H,40,50)(H,41,51)(H4,36,37,42)(H4,38,39,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50084616
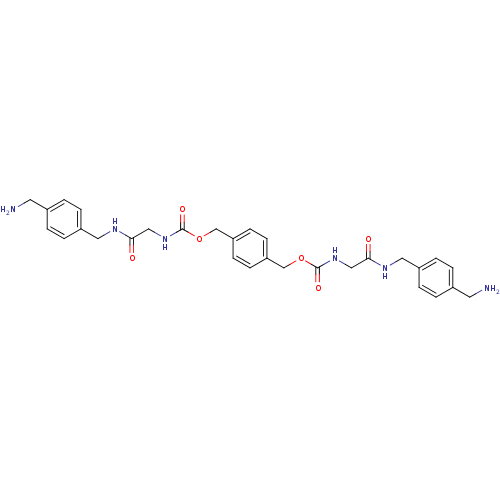
(CHEMBL310290 | [(4-Aminomethyl-benzylcarbamoyl)-me...)Show SMILES NCc1ccc(CNC(=O)CNC(=O)OCc2ccc(COC(=O)NCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N6O6/c31-13-21-1-5-23(6-2-21)15-33-27(37)17-35-29(39)41-19-25-9-11-26(12-10-25)20-42-30(40)36-18-28(38)34-16-24-7-3-22(14-32)4-8-24/h1-12H,13-20,31-32H2,(H,33,37)(H,34,38)(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093178
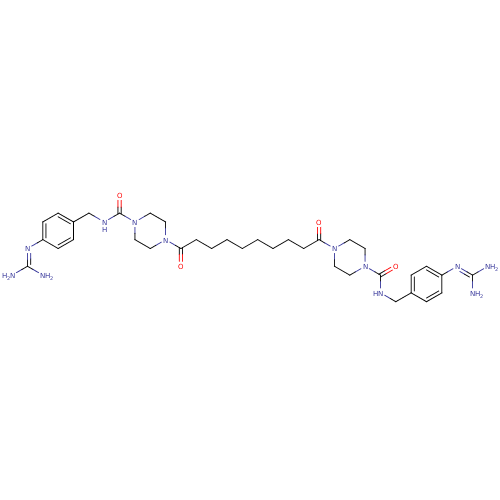
(CHEMBL76883 | Derivative of APC-2059)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C36H54N12O4/c37-33(38)43-29-13-9-27(10-14-29)25-41-35(51)47-21-17-45(18-22-47)31(49)7-5-3-1-2-4-6-8-32(50)46-19-23-48(24-20-46)36(52)42-26-28-11-15-30(16-12-28)44-34(39)40/h9-16H,1-8,17-26H2,(H,41,51)(H,42,52)(H4,37,38,43)(H4,39,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50101011
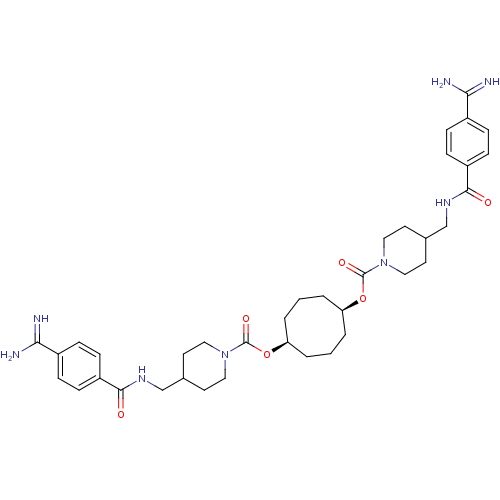
(1,5-di{4-[4-amino(imino)methylphenylcarboxamidomet...)Show SMILES NC(=N)c1ccc(cc1)C(=O)NCC1CCN(CC1)C(=O)O[C@H]1CCC[C@H](CCC1)OC(=O)N1CCC(CNC(=O)c2ccc(cc2)C(N)=N)CC1 Show InChI InChI=1S/C38H52N8O6/c39-33(40)27-7-11-29(12-8-27)35(47)43-23-25-15-19-45(20-16-25)37(49)51-31-3-1-4-32(6-2-5-31)52-38(50)46-21-17-26(18-22-46)24-44-36(48)30-13-9-28(10-14-30)34(41)42/h7-14,25-26,31-32H,1-6,15-24H2,(H3,39,40)(H3,41,42)(H,43,47)(H,44,48)/t31-,32+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of trypsin in human mast cells |
Bioorg Med Chem Lett 11: 1629-33 (2001)
BindingDB Entry DOI: 10.7270/Q2R78DHK |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50101019

(1-{4-[4-amino(imino)methylbenzylcarbamoyl]hexahydr...)Show SMILES NC(=N)c1ccc(CNC(=O)N2CCN(CC2)C(=O)O[C@@H]2CCC[C@@H](CCC2)OC(=O)N2CCN(CC2)C(=O)NCCC2CCNCC2)cc1 Show InChI InChI=1S/C35H55N9O6/c36-31(37)28-9-7-27(8-10-28)25-40-33(46)42-19-23-44(24-20-42)35(48)50-30-5-1-3-29(4-2-6-30)49-34(47)43-21-17-41(18-22-43)32(45)39-16-13-26-11-14-38-15-12-26/h7-10,26,29-30,38H,1-6,11-25H2,(H3,36,37)(H,39,45)(H,40,46)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of trypsin in human mast cells |
Bioorg Med Chem Lett 11: 1629-33 (2001)
BindingDB Entry DOI: 10.7270/Q2R78DHK |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093176

(CHEMBL308763 | Derivative of piperazine-1-carboxyl...)Show SMILES N[C@H]1CC[C@H](CNC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)NCc2ccc(cc2)N=C(N)N)CC1 |wU:22.27,18.18,4.4,wD:1.0,(30.03,-3.88,;28.7,-3.11,;28.68,-1.56,;27.35,-.8,;26.02,-1.57,;24.69,-.8,;23.36,-1.57,;22.03,-.79,;22.03,.75,;20.7,-1.56,;20.7,-3.09,;19.37,-3.86,;18.04,-3.09,;18.04,-1.55,;19.37,-.78,;16.69,-3.86,;16.69,-5.4,;15.36,-3.09,;13.95,-3.69,;13.95,-5.23,;12.86,-6.32,;11.32,-6.32,;10.23,-5.23,;10.23,-3.69,;11.32,-2.61,;12.86,-2.61,;8.8,-5.82,;7.47,-6.59,;7.47,-8.13,;6.14,-5.82,;4.81,-6.59,;3.48,-5.82,;3.48,-4.28,;4.79,-3.51,;6.14,-4.28,;2.13,-3.52,;2.13,-1.98,;.8,-4.29,;-.53,-3.53,;-1.86,-4.3,;-3.2,-3.53,;-4.53,-4.32,;-4.53,-5.86,;-3.2,-6.63,;-1.86,-5.86,;-5.87,-6.63,;-7.2,-5.86,;-7.2,-4.32,;-8.53,-6.63,;26.02,-3.11,;27.35,-3.88,)| Show InChI InChI=1S/C35H56N10O6/c36-27-11-7-25(8-12-27)23-39-32(46)42-15-19-44(20-16-42)34(48)50-29-3-1-5-30(6-2-4-29)51-35(49)45-21-17-43(18-22-45)33(47)40-24-26-9-13-28(14-10-26)41-31(37)38/h9-10,13-14,25,27,29-30H,1-8,11-12,15-24,36H2,(H,39,46)(H,40,47)(H4,37,38,41)/t25-,27-,29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50101016

(1-{4-[4-amino(imino)methylbenzylcarbamoyl]hexahydr...)Show SMILES NC(=N)c1ccc(CNC(=O)N2CCN(CC2)C(=O)O[C@@H]2CCC[C@@H](CCC2)OC(=O)N2CCN(CC2)C(=O)CCCC2CCNCC2)cc1 Show InChI InChI=1S/C36H56N8O6/c37-33(38)29-12-10-28(11-13-29)26-40-34(46)42-20-24-44(25-21-42)36(48)50-31-7-2-5-30(6-3-8-31)49-35(47)43-22-18-41(19-23-43)32(45)9-1-4-27-14-16-39-17-15-27/h10-13,27,30-31,39H,1-9,14-26H2,(H3,37,38)(H,40,46)/t30-,31+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT3 serotonin receptor in NG 108-15 neuroblastoma glioma cells using [3H]-GR-65,630 radioligand. |
Bioorg Med Chem Lett 11: 1629-33 (2001)
BindingDB Entry DOI: 10.7270/Q2R78DHK |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50083549
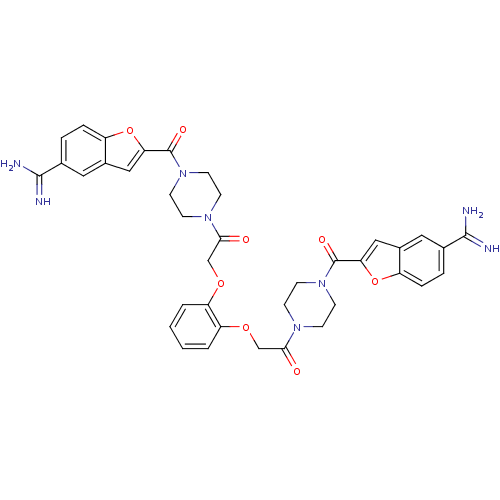
(1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...)Show SMILES NC(=N)c1ccc2oc(cc2c1)C(=O)N1CCN(CC1)C(=O)COc1ccccc1OCC(=O)N1CCN(CC1)C(=O)c1cc2cc(ccc2o1)C(N)=N Show InChI InChI=1S/C38H38N8O8/c39-35(40)23-5-7-27-25(17-23)19-31(53-27)37(49)45-13-9-43(10-14-45)33(47)21-51-29-3-1-2-4-30(29)52-22-34(48)44-11-15-46(16-12-44)38(50)32-20-26-18-24(36(41)42)6-8-28(26)54-32/h1-8,17-20H,9-16,21-22H2,(H3,39,40)(H3,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD.
Curated by ChEMBL
| Assay Description
Inhibition of tryptase activity |
Bioorg Med Chem Lett 9: 3285-90 (2000)
BindingDB Entry DOI: 10.7270/Q2TX3DK3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156461
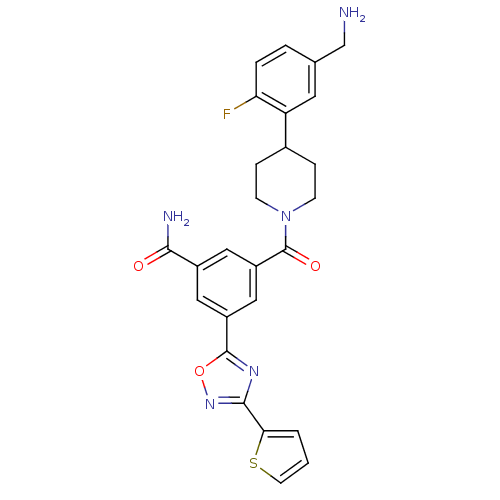
(3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H24FN5O3S/c27-21-4-3-15(14-28)10-20(21)16-5-7-32(8-6-16)26(34)19-12-17(23(29)33)11-18(13-19)25-30-24(31-35-25)22-2-1-9-36-22/h1-4,9-13,16H,5-8,14,28H2,(H2,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187167
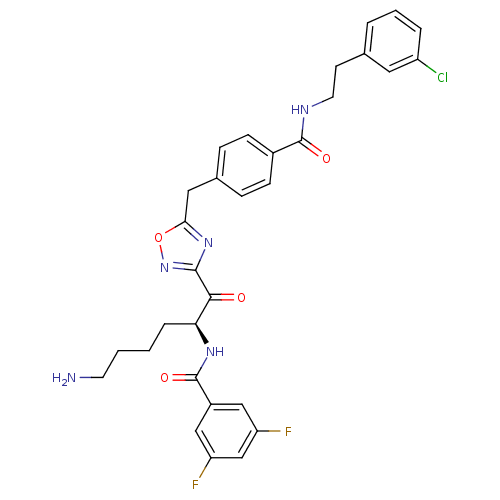
(CHEMBL211357 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1cc(F)cc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(14-23)11-13-36-30(41)21-9-7-20(8-10-21)15-27-38-29(39-43-27)28(40)26(6-1-2-12-35)37-31(42)22-16-24(33)18-25(34)17-22/h3-5,7-10,14,16-18,26H,1-2,6,11-13,15,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156460
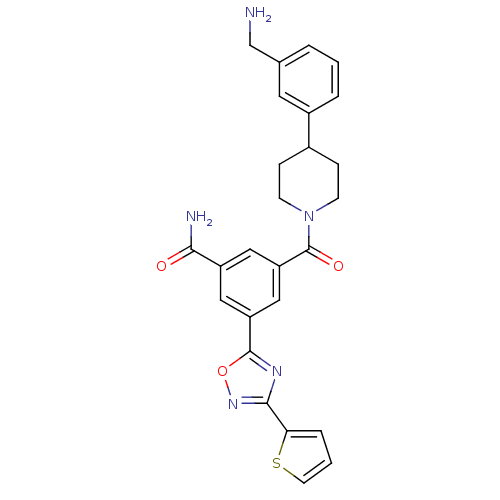
(3-[4-(3-Aminomethyl-phenyl)-piperidine-1-carbonyl]...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H25N5O3S/c27-15-16-3-1-4-18(11-16)17-6-8-31(9-7-17)26(33)21-13-19(23(28)32)12-20(14-21)25-29-24(30-34-25)22-5-2-10-35-22/h1-5,10-14,17H,6-9,15,27H2,(H2,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187166
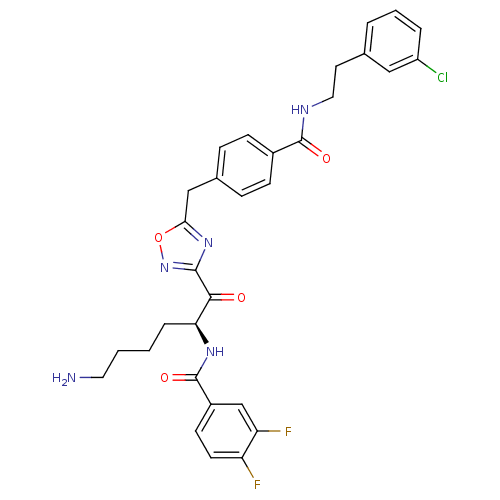
(CHEMBL380293 | N-[(S)-5-amino-1-(5-{4-[2-(3-chloro...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H30ClF2N5O4/c32-23-5-3-4-19(16-23)13-15-36-30(41)21-9-7-20(8-10-21)17-27-38-29(39-43-27)28(40)26(6-1-2-14-35)37-31(42)22-11-12-24(33)25(34)18-22/h3-5,7-12,16,18,26H,1-2,6,13-15,17,35H2,(H,36,41)(H,37,42)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
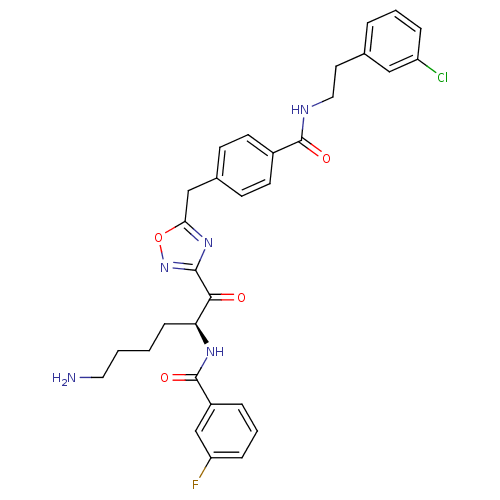
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187177
(CHEMBL212774 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1cccc(F)c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31ClFN5O4/c32-24-7-3-5-20(17-24)14-16-35-30(40)22-12-10-21(11-13-22)18-27-37-29(38-42-27)28(39)26(9-1-2-15-34)36-31(41)23-6-4-8-25(33)19-23/h3-8,10-13,17,19,26H,1-2,9,14-16,18,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
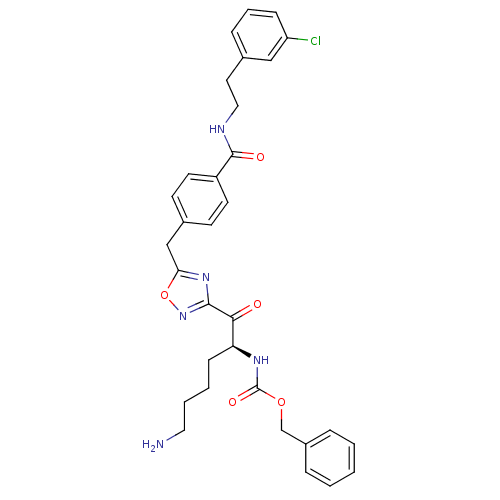
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187163
((S)-benzyl 1-(5-(4-((3-chlorophenethyl)carbamoyl)b...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C32H34ClN5O5/c33-26-10-6-9-22(19-26)16-18-35-31(40)25-14-12-23(13-15-25)20-28-37-30(38-43-28)29(39)27(11-4-5-17-34)36-32(41)42-21-24-7-2-1-3-8-24/h1-3,6-10,12-15,19,27H,4-5,11,16-18,20-21,34H2,(H,35,40)(H,36,41)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187160

(CHEMBL213928 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31ClFN5O4/c32-24-5-3-4-20(18-24)15-17-35-30(40)22-9-7-21(8-10-22)19-27-37-29(38-42-27)28(39)26(6-1-2-16-34)36-31(41)23-11-13-25(33)14-12-23/h3-5,7-14,18,26H,1-2,6,15-17,19,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
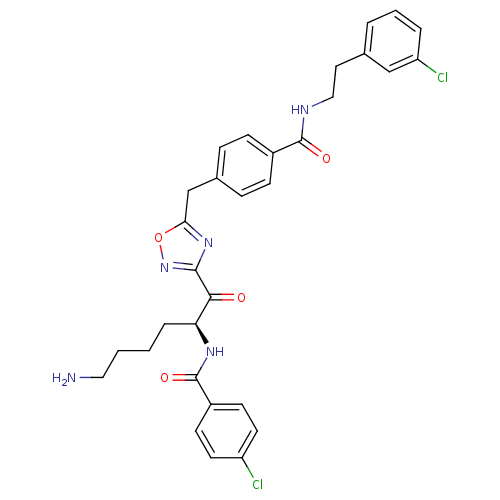
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187168
(CHEMBL377656 | N-[(2S)-6-amino-1-{5-[(4-{[2-(3-chl...)Show SMILES NCCCC[C@H](NC(=O)c1ccc(Cl)cc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C31H31Cl2N5O4/c32-24-13-11-23(12-14-24)31(41)36-26(6-1-2-16-34)28(39)29-37-27(42-38-29)19-21-7-9-22(10-8-21)30(40)35-17-15-20-4-3-5-25(33)18-20/h3-5,7-14,18,26H,1-2,6,15-17,19,34H2,(H,35,40)(H,36,41)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187176
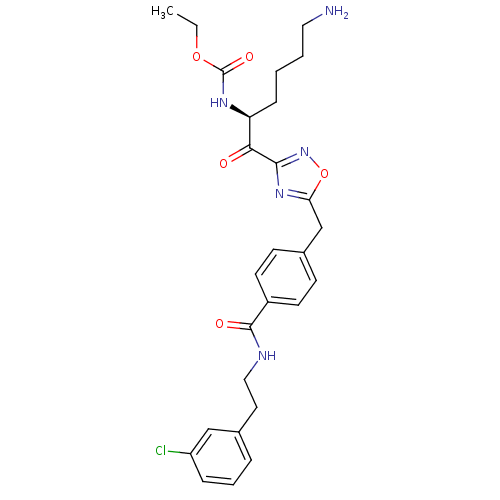
((S)-ethyl 1-(5-(4-((3-chlorophenethyl)carbamoyl)be...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C27H32ClN5O5/c1-2-37-27(36)31-22(8-3-4-14-29)24(34)25-32-23(38-33-25)17-19-9-11-20(12-10-19)26(35)30-15-13-18-6-5-7-21(28)16-18/h5-7,9-12,16,22H,2-4,8,13-15,17,29H2,1H3,(H,30,35)(H,31,36)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093186

(CHEMBL76424 | Derivative of piperazine-1-carboxyli...)Show SMILES NC(=N)N1CCC(CCC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)CCC2CCN(CC2)C(N)=N)CC1 Show InChI InChI=1S/C36H62N10O6/c37-33(38)43-15-11-27(12-16-43)7-9-31(47)41-19-23-45(24-20-41)35(49)51-29-3-1-4-30(6-2-5-29)52-36(50)46-25-21-42(22-26-46)32(48)10-8-28-13-17-44(18-14-28)34(39)40/h27-30H,1-26H2,(H3,37,38)(H3,39,40)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093197
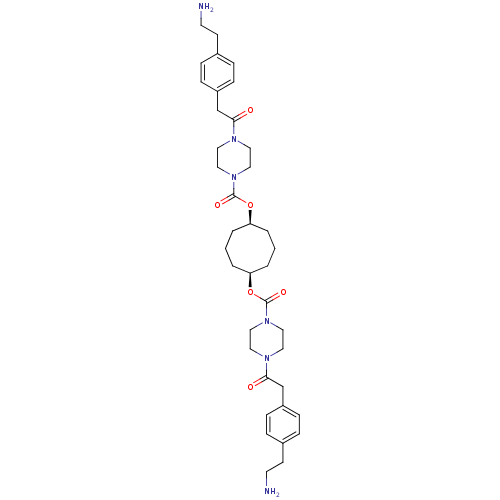
(CHEMBL75679 | Derivative of piperazine-1-carboxyli...)Show SMILES NCCc1ccc(CC(=O)N2CCN(CC2)C(=O)O[C@H]2CCC[C@H](CCC2)OC(=O)N2CCN(CC2)C(=O)Cc2ccc(CCN)cc2)cc1 Show InChI InChI=1S/C38H54N6O6/c39-17-15-29-7-11-31(12-8-29)27-35(45)41-19-23-43(24-20-41)37(47)49-33-3-1-4-34(6-2-5-33)50-38(48)44-25-21-42(22-26-44)36(46)28-32-13-9-30(10-14-32)16-18-40/h7-14,33-34H,1-6,15-28,39-40H2/t33-,34+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187162
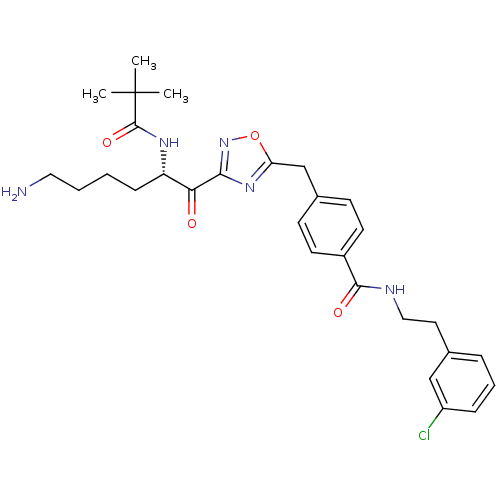
((S)-N-(3-chlorophenethyl)-4-((3-(6-amino-2-pivalam...)Show SMILES CC(C)(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C29H36ClN5O4/c1-29(2,3)28(38)33-23(9-4-5-15-31)25(36)26-34-24(39-35-26)18-20-10-12-21(13-11-20)27(37)32-16-14-19-7-6-8-22(30)17-19/h6-8,10-13,17,23H,4-5,9,14-16,18,31H2,1-3H3,(H,32,37)(H,33,38)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093132
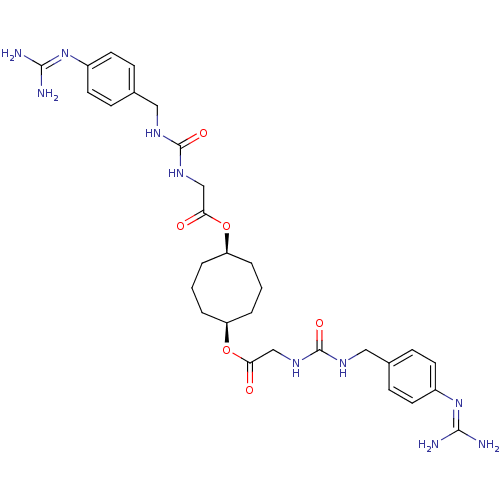
(CHEMBL75749 | [3-(4-Guanidino-benzyl)-ureido]-acet...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C30H42N10O6/c31-27(32)39-21-11-7-19(8-12-21)15-35-29(43)37-17-25(41)45-23-3-1-4-24(6-2-5-23)46-26(42)18-38-30(44)36-16-20-9-13-22(14-10-20)40-28(33)34/h7-14,23-24H,1-6,15-18H2,(H4,31,32,39)(H4,33,34,40)(H2,35,37,43)(H2,36,38,44)/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187182

((S)-N-(1-(5-(4-((3-chlorophenethyl)carbamoyl)benzy...)Show SMILES NCCCC[C@H](NC(=O)c1ccc2OCOc2c1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccc(Cl)c2)n1 Show InChI InChI=1S/C32H32ClN5O6/c33-24-5-3-4-20(16-24)13-15-35-31(40)22-9-7-21(8-10-22)17-28-37-30(38-44-28)29(39)25(6-1-2-14-34)36-32(41)23-11-12-26-27(18-23)43-19-42-26/h3-5,7-12,16,18,25H,1-2,6,13-15,17,19,34H2,(H,35,40)(H,36,41)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411015
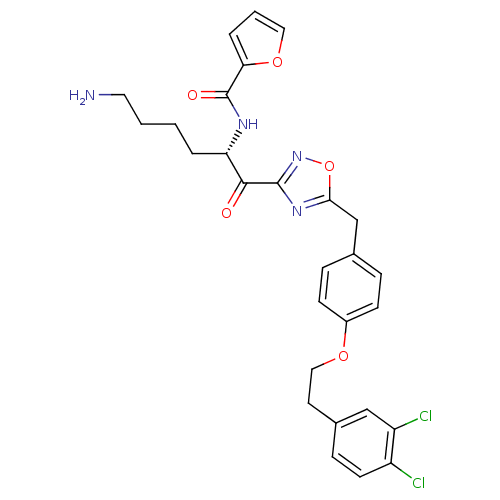
(CHEMBL539086)Show SMILES NCCCC[C@H](NC(=O)c1ccco1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H28Cl2N4O5/c29-21-11-8-19(16-22(21)30)12-15-37-20-9-6-18(7-10-20)17-25-33-27(34-39-25)26(35)23(4-1-2-13-31)32-28(36)24-5-3-14-38-24/h3,5-11,14,16,23H,1-2,4,12-13,15,17,31H2,(H,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187164
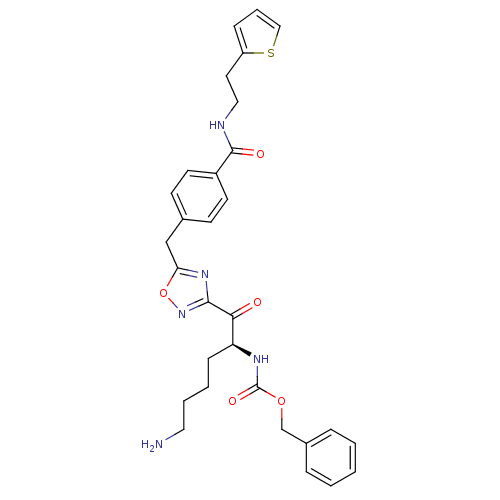
((S)-benzyl 1-(5-(4-((2-(thiophen-2-yl)ethyl)carbam...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCc2cccs2)n1 Show InChI InChI=1S/C30H33N5O5S/c31-16-5-4-10-25(33-30(38)39-20-22-7-2-1-3-8-22)27(36)28-34-26(40-35-28)19-21-11-13-23(14-12-21)29(37)32-17-15-24-9-6-18-41-24/h1-3,6-9,11-14,18,25H,4-5,10,15-17,19-20,31H2,(H,32,37)(H,33,38)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411006
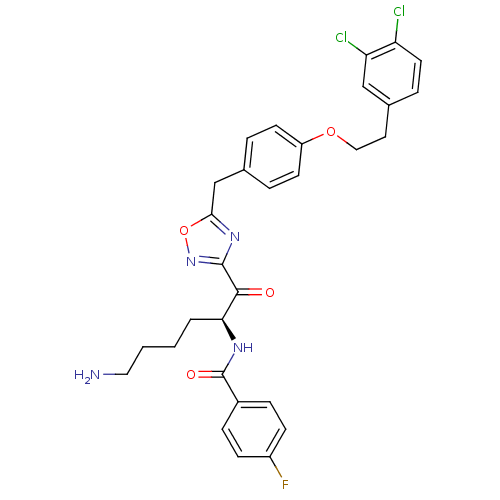
(CHEMBL539088)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-24-13-6-20(17-25(24)32)14-16-40-23-11-4-19(5-12-23)18-27-36-29(37-41-27)28(38)26(3-1-2-15-34)35-30(39)21-7-9-22(33)10-8-21/h4-13,17,26H,1-3,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM14312
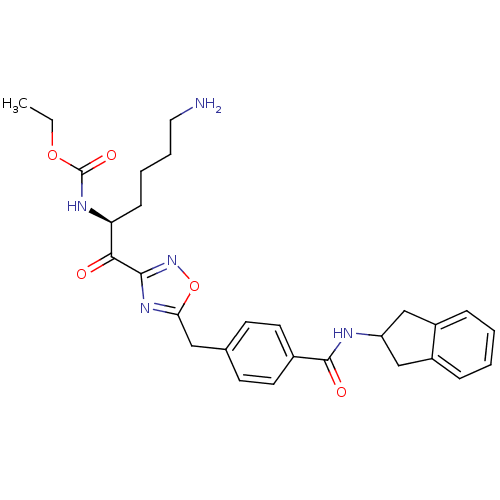
(CHEMBL214368 | CRA23 | ethyl N-[(2S)-6-amino-1-(5-...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(cc2)C(=O)NC2Cc3ccccc3C2)n1 |r| Show InChI InChI=1S/C28H33N5O5/c1-2-37-28(36)31-23(9-5-6-14-29)25(34)26-32-24(38-33-26)15-18-10-12-19(13-11-18)27(35)30-22-16-20-7-3-4-8-21(20)17-22/h3-4,7-8,10-13,22-23H,2,5-6,9,14-17,29H2,1H3,(H,30,35)(H,31,36)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411011
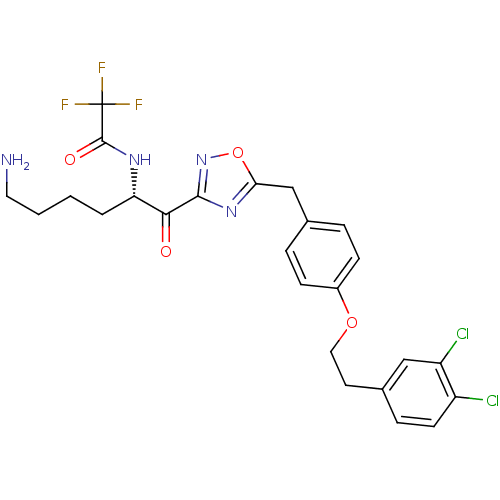
(CHEMBL537868)Show SMILES NCCCC[C@H](NC(=O)C(F)(F)F)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C25H25Cl2F3N4O4/c26-18-9-6-16(13-19(18)27)10-12-37-17-7-4-15(5-8-17)14-21-33-23(34-38-21)22(35)20(3-1-2-11-31)32-24(36)25(28,29)30/h4-9,13,20H,1-3,10-12,14,31H2,(H,32,36)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187169
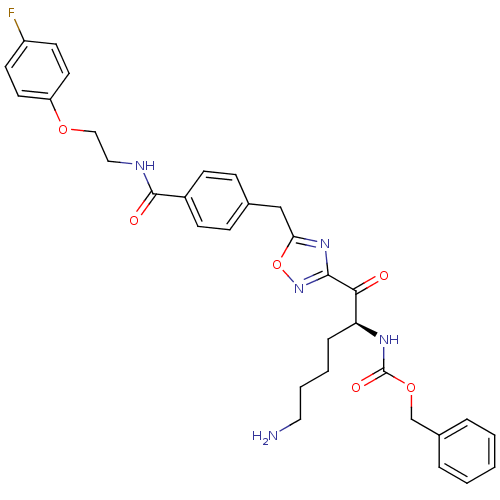
((S)-benzyl 1-(5-(4-((2-(4-fluorophenoxy)ethyl)carb...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCOc2ccc(F)cc2)n1 Show InChI InChI=1S/C32H34FN5O6/c33-25-13-15-26(16-14-25)42-19-18-35-31(40)24-11-9-22(10-12-24)20-28-37-30(38-44-28)29(39)27(8-4-5-17-34)36-32(41)43-21-23-6-2-1-3-7-23/h1-3,6-7,9-16,27H,4-5,8,17-21,34H2,(H,35,40)(H,36,41)/t27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093198
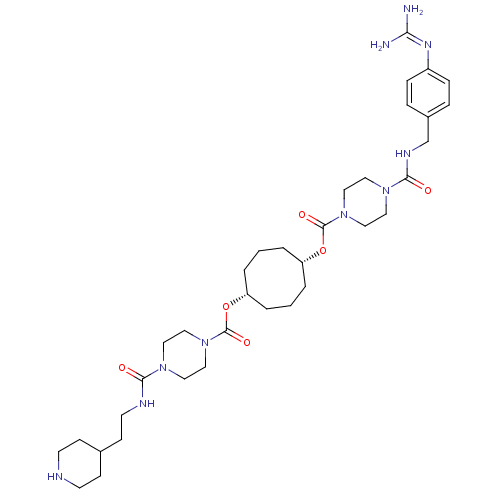
(CHEMBL311043 | Derivative of piperazine-1-carboxyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@@H]-2-[#6]-[#6]-[#6]-[#6@@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-2-[#6]-[#6]-[#7]-[#6]-[#6]-2)cc1 Show InChI InChI=1S/C35H56N10O6/c36-31(37)41-28-9-7-27(8-10-28)25-40-33(47)43-19-23-45(24-20-43)35(49)51-30-5-1-3-29(4-2-6-30)50-34(48)44-21-17-42(18-22-44)32(46)39-16-13-26-11-14-38-15-12-26/h7-10,26,29-30,38H,1-6,11-25H2,(H,39,46)(H,40,47)(H4,36,37,41)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50093164
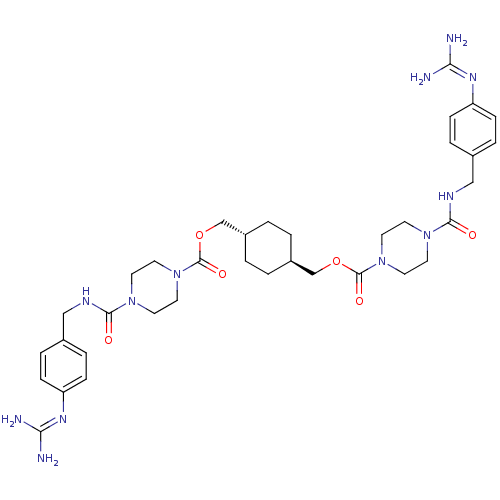
(CHEMBL308632 | Derivative of APC-2059)Show SMILES NC(N)=Nc1ccc(CNC(=O)N2CCN(CC2)C(=O)OC[C@H]2CC[C@H](COC(=O)N3CCN(CC3)C(=O)NCc3ccc(cc3)N=C(N)N)CC2)cc1 |wU:25.26,wD:22.22,(-5.9,-4.38,;-5.9,-5.92,;-7.23,-6.69,;-4.57,-6.69,;-3.22,-5.92,;-1.89,-6.69,;-.55,-5.92,;-.55,-4.38,;.78,-3.6,;2.11,-4.35,;3.44,-3.58,;3.44,-2.04,;4.79,-4.35,;6.1,-3.57,;7.45,-4.34,;7.45,-5.89,;6.12,-6.66,;4.79,-5.89,;8.79,-6.66,;8.79,-8.18,;10.12,-5.89,;11.45,-6.66,;12.78,-5.89,;14.12,-6.66,;15.45,-5.89,;15.46,-4.34,;16.79,-3.57,;18.13,-4.34,;19.46,-5.12,;19.46,-6.66,;20.8,-4.34,;22.13,-5.12,;23.46,-4.35,;23.46,-2.81,;22.14,-2.03,;20.8,-2.8,;24.81,-2.04,;24.81,-.5,;26.14,-2.81,;27.48,-2.06,;28.81,-2.83,;28.81,-4.38,;30.14,-5.15,;31.47,-4.38,;31.47,-2.83,;30.14,-2.06,;32.82,-5.15,;34.15,-4.38,;34.15,-2.83,;35.49,-5.15,;14.12,-3.57,;12.78,-4.35,;-1.89,-3.6,;-3.22,-4.38,)| Show InChI InChI=1S/C36H52N12O6/c37-31(38)43-29-9-5-25(6-10-29)21-41-33(49)45-13-17-47(18-14-45)35(51)53-23-27-1-2-28(4-3-27)24-54-36(52)48-19-15-46(16-20-48)34(50)42-22-26-7-11-30(12-8-26)44-32(39)40/h5-12,27-28H,1-4,13-24H2,(H,41,49)(H,42,50)(H4,37,38,43)(H4,39,40,44)/t27-,28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for its inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2361-6 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BWR |
More data for this
Ligand-Target Pair | |
Dimer of Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187179
((S)-benzyl 1-(5-(4-((3-phenylpropyl)carbamoyl)benz...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)c1noc(Cc2ccc(cc2)C(=O)NCCCc2ccccc2)n1 Show InChI InChI=1S/C33H37N5O5/c34-20-8-7-15-28(36-33(41)42-23-26-12-5-2-6-13-26)30(39)31-37-29(43-38-31)22-25-16-18-27(19-17-25)32(40)35-21-9-14-24-10-3-1-4-11-24/h1-6,10-13,16-19,28H,7-9,14-15,20-23,34H2,(H,35,40)(H,36,41)/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of human beta tryptase |
Bioorg Med Chem Lett 16: 4036-40 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.009
BindingDB Entry DOI: 10.7270/Q20864ZV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data