

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50437999 (CHEMBL2409452) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human pancreatic chymotrypsin using Suc-Ala-Ala-Pro-7-amino-4-methylcoumarin as substrate by fluorescence microplate reader analysis | J Med Chem 56: 6259-72 (2013) Article DOI: 10.1021/jm400742j BindingDB Entry DOI: 10.7270/Q2MC91FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50437997 (CHEMBL2409457) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human pancreatic chymotrypsin using Suc-Ala-Ala-Pro-7-amino-4-methylcoumarin as substrate by fluorescence microplate reader analysis | J Med Chem 56: 6259-72 (2013) Article DOI: 10.1021/jm400742j BindingDB Entry DOI: 10.7270/Q2MC91FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50438000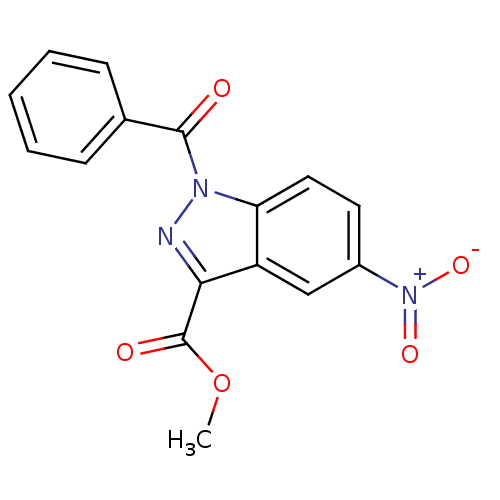 (CHEMBL2409451) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human pancreatic chymotrypsin using Suc-Ala-Ala-Pro-7-amino-4-methylcoumarin as substrate by fluorescence microplate reader analysis | J Med Chem 56: 6259-72 (2013) Article DOI: 10.1021/jm400742j BindingDB Entry DOI: 10.7270/Q2MC91FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50437992 (CHEMBL2409445) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human pancreatic chymotrypsin using Suc-Ala-Ala-Pro-7-amino-4-methylcoumarin as substrate by fluorescence microplate reader analysis | J Med Chem 56: 6259-72 (2013) Article DOI: 10.1021/jm400742j BindingDB Entry DOI: 10.7270/Q2MC91FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50437998 (CHEMBL2409456) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human pancreatic chymotrypsin using Suc-Ala-Ala-Pro-7-amino-4-methylcoumarin as substrate by fluorescence microplate reader analysis | J Med Chem 56: 6259-72 (2013) Article DOI: 10.1021/jm400742j BindingDB Entry DOI: 10.7270/Q2MC91FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50437996 (CHEMBL2409458) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human pancreatic chymotrypsin using Suc-Ala-Ala-Pro-7-amino-4-methylcoumarin as substrate by fluorescence microplate reader analysis | J Med Chem 56: 6259-72 (2013) Article DOI: 10.1021/jm400742j BindingDB Entry DOI: 10.7270/Q2MC91FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50437991 (CHEMBL2409450) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human pancreatic chymotrypsin using Suc-Ala-Ala-Pro-7-amino-4-methylcoumarin as substrate by fluorescence microplate reader analysis | J Med Chem 56: 6259-72 (2013) Article DOI: 10.1021/jm400742j BindingDB Entry DOI: 10.7270/Q2MC91FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50437994 (CHEMBL2409571) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human pancreatic chymotrypsin using Suc-Ala-Ala-Pro-7-amino-4-methylcoumarin as substrate by fluorescence microplate reader analysis | J Med Chem 56: 6259-72 (2013) Article DOI: 10.1021/jm400742j BindingDB Entry DOI: 10.7270/Q2MC91FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50437995 (CHEMBL2409570) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human pancreatic chymotrypsin using Suc-Ala-Ala-Pro-7-amino-4-methylcoumarin as substrate by fluorescence microplate reader analysis | J Med Chem 56: 6259-72 (2013) Article DOI: 10.1021/jm400742j BindingDB Entry DOI: 10.7270/Q2MC91FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50437993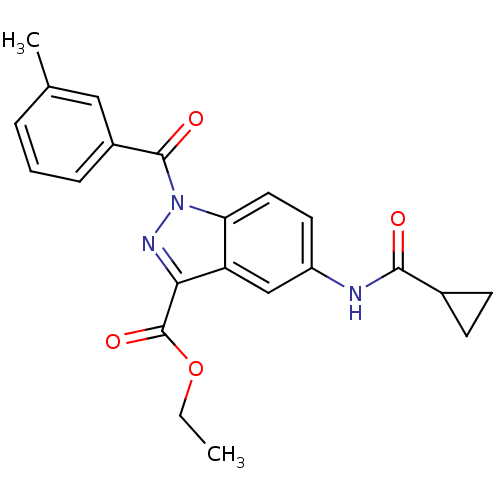 (CHEMBL2409575) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human pancreatic chymotrypsin using Suc-Ala-Ala-Pro-7-amino-4-methylcoumarin as substrate by fluorescence microplate reader analysis | J Med Chem 56: 6259-72 (2013) Article DOI: 10.1021/jm400742j BindingDB Entry DOI: 10.7270/Q2MC91FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM2581 (3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50111589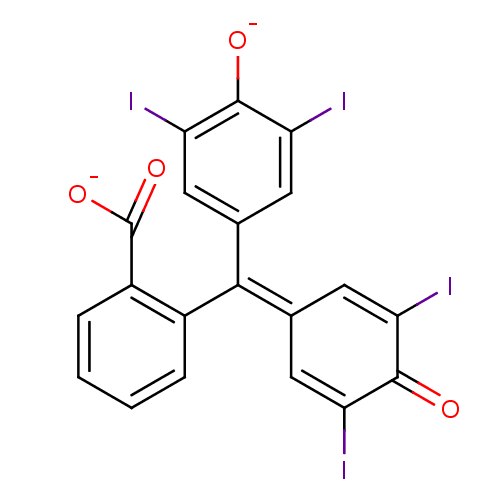 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50111604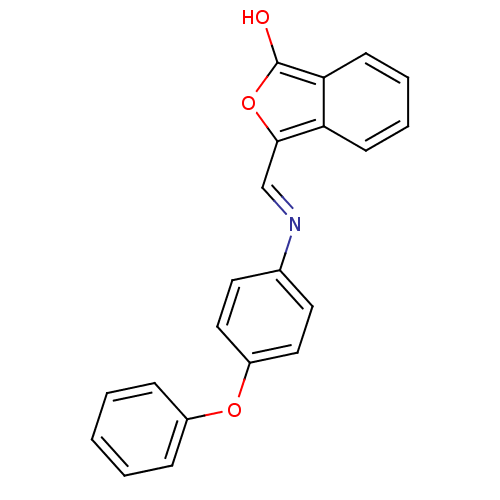 (3-[(4-Phenoxy-phenylamino)-methylene]-3H-isobenzof...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM3175 (3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50111585 (4-(4-Bromo-phenylazo)-phenol | 4-bromophenylazophe...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50126829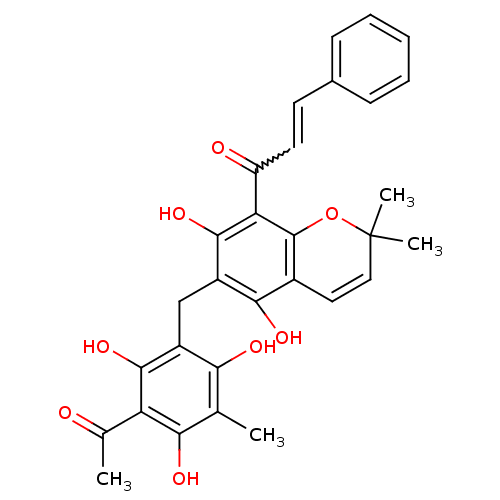 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50055218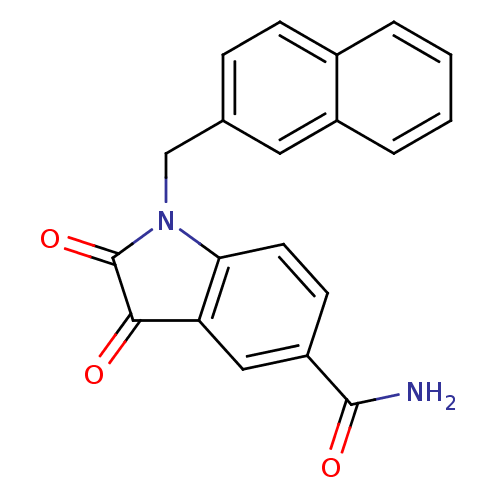 (1-(2-naphthlmethyl) isatin-5-carboxamide | 1-Napht...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin | J Med Chem 49: 3440-3 (2006) Article DOI: 10.1021/jm0602357 BindingDB Entry DOI: 10.7270/Q2TD9WZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50310357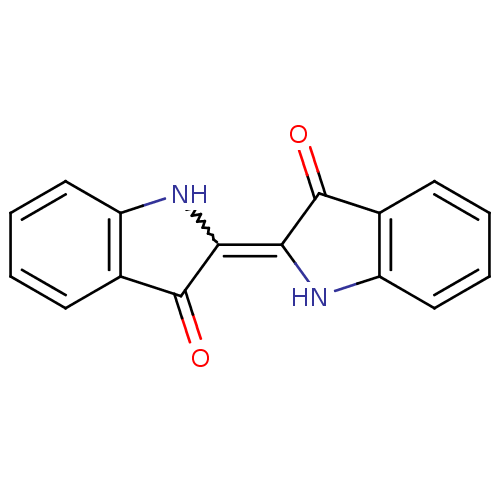 (CHEMBL599552 | indigo) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50126827 (4-(1-Hydroxy-naphthalen-2-ylazo)-naphthalene-1-sul...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50111591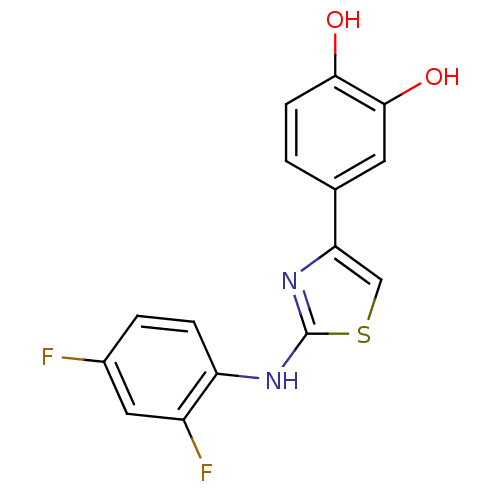 (4-[2-(2,4-Difluoro-phenylamino)-thiazol-4-yl]-benz...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50023867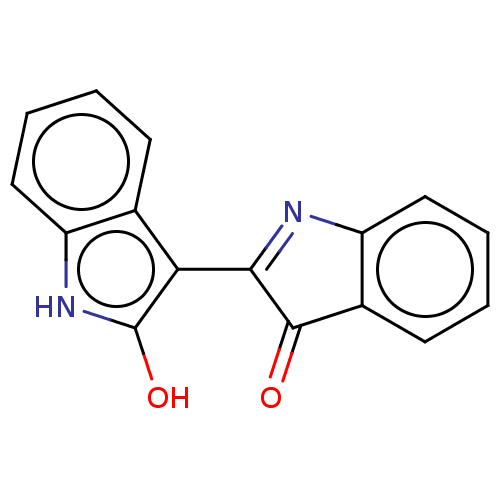 (Indirubin) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50072147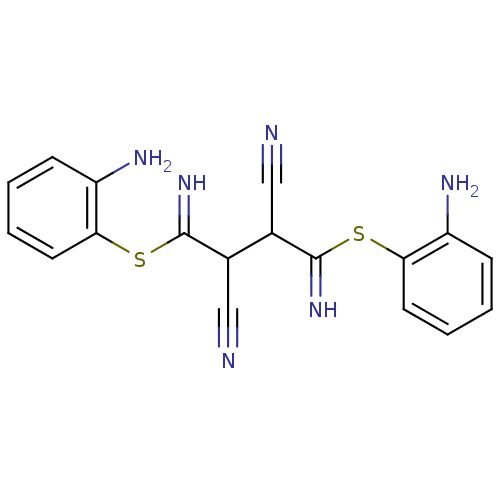 ((2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM2681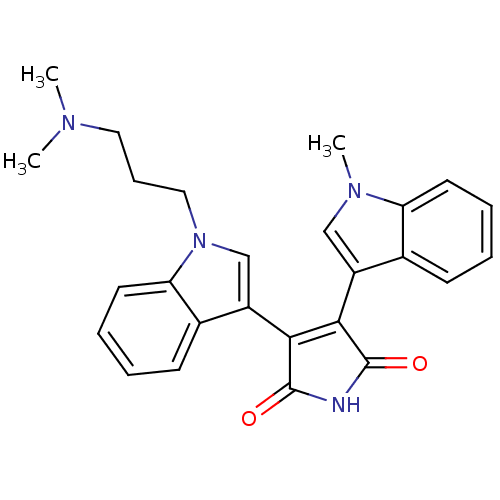 (3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||