 Found 15 hits of ki data for polymerid = 50001044
Found 15 hits of ki data for polymerid = 50001044 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50284625
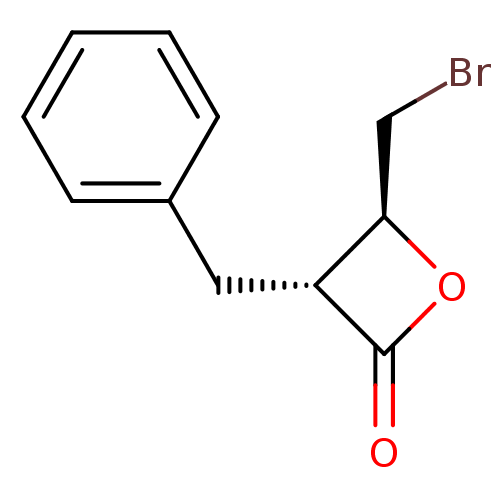
((3R,4S)-3-Benzyl-4-bromomethyl-oxetan-2-one | CHEM...)Show InChI InChI=1S/C11H11BrO2/c12-7-10-9(11(13)14-10)6-8-4-2-1-3-5-8/h1-5,9-10H,6-7H2/t9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Alpha-chymotrypsin was determined by competitive substrate assay method |
Bioorg Med Chem Lett 5: 1287-1292 (1995)
Article DOI: 10.1016/0960-894X(95)00216-G
BindingDB Entry DOI: 10.7270/Q2H70FRX |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50175075
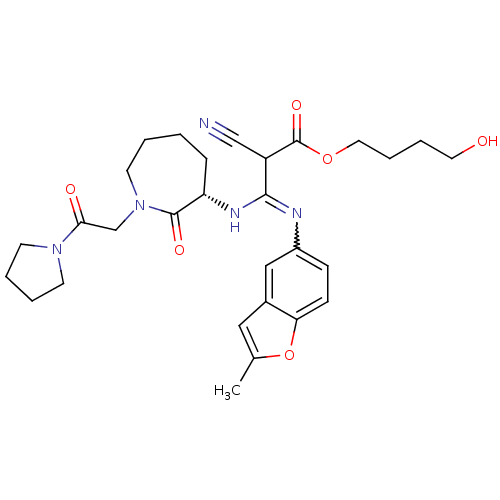
((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to chymotrypsin |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM12657
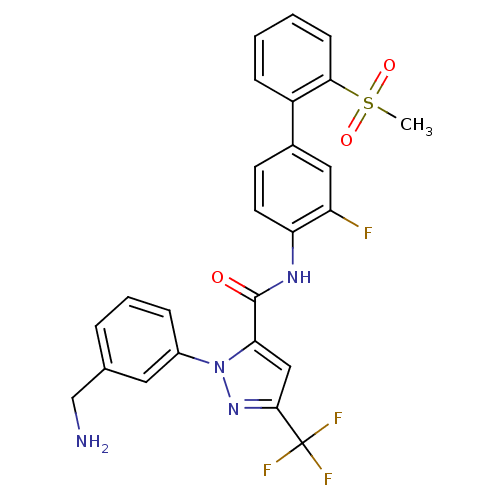
(1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(CN)c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H20F4N4O3S/c1-37(35,36)22-8-3-2-7-18(22)16-9-10-20(19(26)12-16)31-24(34)21-13-23(25(27,28)29)32-33(21)17-6-4-5-15(11-17)14-30/h2-13H,14,30H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro activity against human Chymotrypsinogen B1 |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM12657

(1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(CN)c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H20F4N4O3S/c1-37(35,36)22-8-3-2-7-18(22)16-9-10-20(19(26)12-16)31-24(34)21-13-23(25(27,28)29)32-33(21)17-6-4-5-15(11-17)14-30/h2-13H,14,30H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Tested in vitro for inhibition of human Chymotrypsinogen |
J Med Chem 46: 5298-315 (2003)
Article DOI: 10.1021/jm030212h
BindingDB Entry DOI: 10.7270/Q2ZW1MP2 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50152752
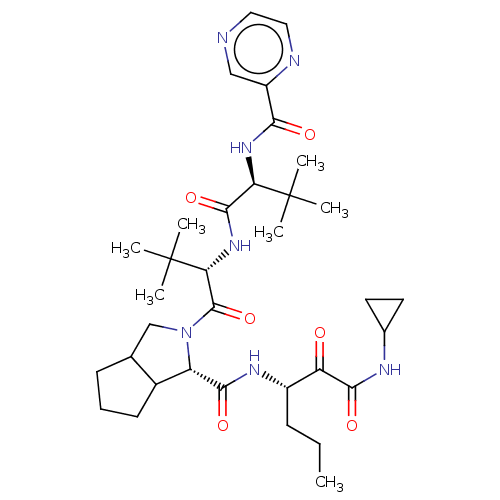
((S)-2-((S)-2-{(S)-3,3-Dimethyl-2-[(pyrazine-2-carb...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C34H51N7O6/c1-8-10-22(25(42)30(45)37-20-13-14-20)38-29(44)24-21-12-9-11-19(21)18-41(24)32(47)27(34(5,6)7)40-31(46)26(33(2,3)4)39-28(43)23-17-35-15-16-36-23/h15-17,19-22,24,26-27H,8-14,18H2,1-7H3,(H,37,45)(H,38,44)(H,39,43)(H,40,46)/t19?,21?,22?,24-,26+,27+/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards human Chymotrypsinogen |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50152748
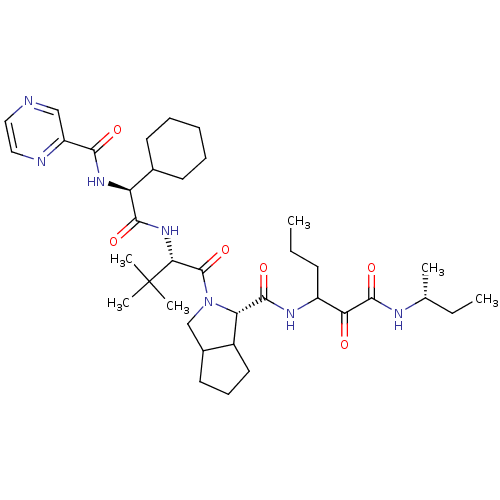
((S)-2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carb...)Show SMILES CCCC(NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)N[C@H](C)CC Show InChI InChI=1S/C37H57N7O6/c1-7-13-26(30(45)35(49)40-22(3)8-2)41-34(48)29-25-17-12-16-24(25)21-44(29)36(50)31(37(4,5)6)43-33(47)28(23-14-10-9-11-15-23)42-32(46)27-20-38-18-19-39-27/h18-20,22-26,28-29,31H,7-17,21H2,1-6H3,(H,40,49)(H,41,48)(H,42,46)(H,43,47)/t22-,24?,25?,26?,28+,29+,31-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards human Chymotrypsinogen |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50152755
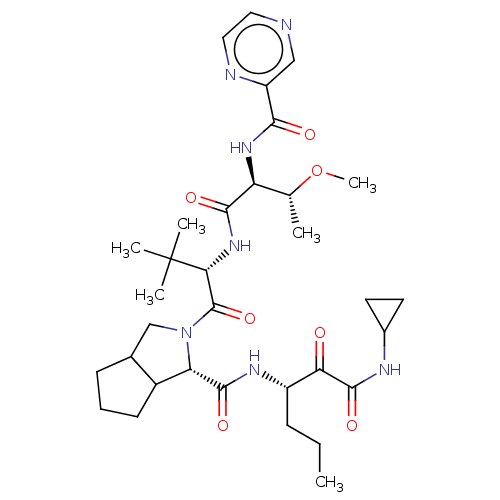
(2-((S)-2-{(S)-3-Methoxy-2-[(pyrazine-2-carbonyl)-a...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)[C@@H](C)OC)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O7/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(3,4)5)39-29(43)24(18(2)47-6)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t18?,19?,21?,22?,24-,25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards human Chymotrypsinogen |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50152753

((S)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[(pyrazi...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)[C@@H](C)CC)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C34H51N7O6/c1-7-10-23(27(42)32(46)37-21-13-14-21)38-31(45)26-22-12-9-11-20(22)18-41(26)33(47)28(34(4,5)6)40-30(44)25(19(3)8-2)39-29(43)24-17-35-15-16-36-24/h15-17,19-23,25-26,28H,7-14,18H2,1-6H3,(H,37,46)(H,38,45)(H,39,43)(H,40,44)/t19?,20?,22?,23?,25-,26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards human Chymotrypsinogen |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50152754
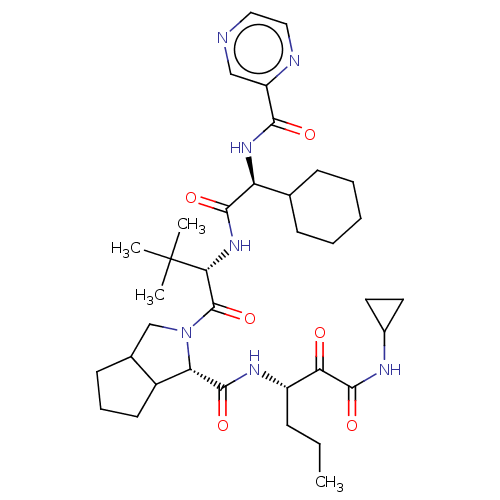
(2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carbonyl...)Show SMILES CCC[C@H](NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C36H53N7O6/c1-5-10-25(29(44)34(48)39-23-15-16-23)40-33(47)28-24-14-9-13-22(24)20-43(28)35(49)30(36(2,3)4)42-32(46)27(21-11-7-6-8-12-21)41-31(45)26-19-37-17-18-38-26/h17-19,21-25,27-28,30H,5-16,20H2,1-4H3,(H,39,48)(H,40,47)(H,41,45)(H,42,46)/t22?,24?,25?,27-,28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards human Chymotrypsinogen |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50152750
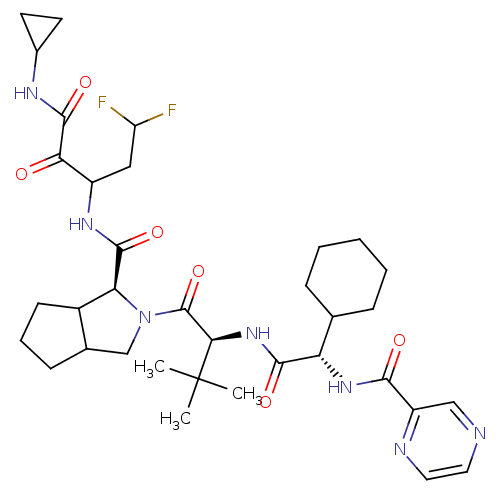
(2-((S)-2-{(S)-2-Cyclohexyl-2-[(pyrazine-2-carbonyl...)Show SMILES CC(C)(C)[C@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(=O)N1CC2CCCC2[C@H]1C(=O)NC(CC(F)F)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C35H49F2N7O6/c1-35(2,3)29(43-31(47)26(19-8-5-4-6-9-19)42-30(46)24-17-38-14-15-39-24)34(50)44-18-20-10-7-11-22(20)27(44)32(48)41-23(16-25(36)37)28(45)33(49)40-21-12-13-21/h14-15,17,19-23,25-27,29H,4-13,16,18H2,1-3H3,(H,40,49)(H,41,48)(H,42,46)(H,43,47)/t20?,22?,23?,26-,27-,29+/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards human Chymotrypsinogen |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50152751

(2-((S)-2-{(S)-2-Cyclohexyl-2-[((R)-pyrazine-2-carb...)Show SMILES CCCC(NC(=O)[C@@H]1C2CCCC2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C1CCCCC1)C(C)(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C41H57N7O6/c1-6-14-30(34(49)39(53)44-25(2)26-15-9-7-10-16-26)45-38(52)33-29-20-13-19-28(29)24-48(33)40(54)35(41(3,4)5)47-37(51)32(27-17-11-8-12-18-27)46-36(50)31-23-42-21-22-43-31/h7,9-10,15-16,21-23,25,27-30,32-33,35H,6,8,11-14,17-20,24H2,1-5H3,(H,44,53)(H,45,52)(H,46,50)(H,47,51)/t25-,28?,29?,30?,32+,33+,35-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards human Chymotrypsinogen |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50137733
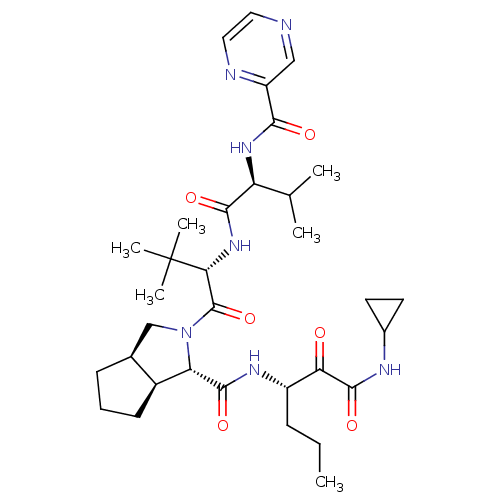
((1S,5S,6R)-2-((S)-3,3-Dimethyl-2-{(S)-3-methyl-2-[...)Show SMILES CCC[C@H](NC(=O)[C@@H]1[C@H]2CCC[C@H]2CN1C(=O)[C@@H](NC(=O)[C@@H](NC(=O)c1cnccn1)C(C)C)C(C)(C)C)C(=O)C(=O)NC1CC1 Show InChI InChI=1S/C33H49N7O6/c1-7-9-22(26(41)31(45)36-20-12-13-20)37-30(44)25-21-11-8-10-19(21)17-40(25)32(46)27(33(4,5)6)39-29(43)24(18(2)3)38-28(42)23-16-34-14-15-35-23/h14-16,18-22,24-25,27H,7-13,17H2,1-6H3,(H,36,45)(H,37,44)(H,38,42)(H,39,43)/t19-,21-,22-,24-,25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratory
Curated by ChEMBL
| Assay Description
Binding affinity towards human Chymotrypsinogen |
Bioorg Med Chem Lett 14: 5007-11 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.007
BindingDB Entry DOI: 10.7270/Q29P314N |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50410995
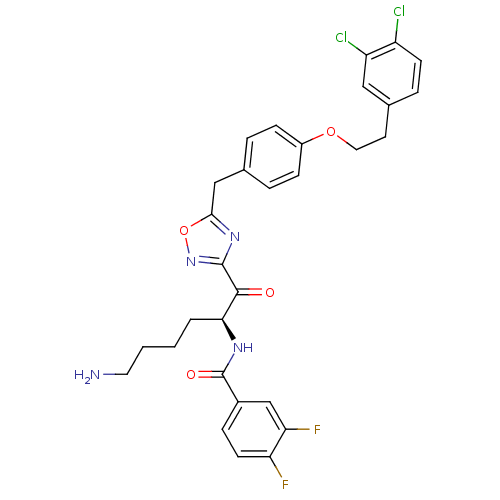
(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Binding affinity to chymotrypsin |
Bioorg Med Chem Lett 16: 4085-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.088
BindingDB Entry DOI: 10.7270/Q2X929W7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data