 Found 1116 hits of ic50 data for polymerid = 50001129,6095
Found 1116 hits of ic50 data for polymerid = 50001129,6095 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50423388
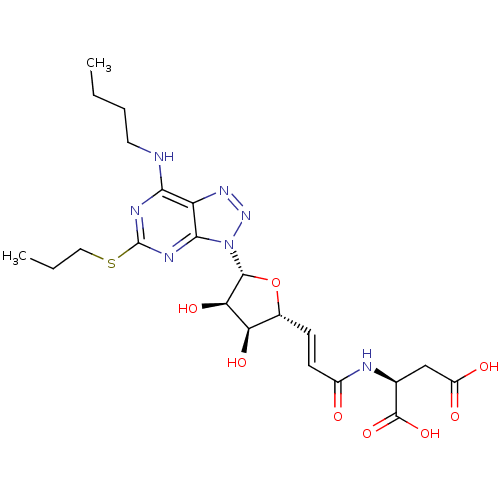
(CHEMBL251024)Show SMILES CCCCNc1nc(SCCC)nc2n(nnc12)[C@@H]1O[C@H](\C=C\C(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C22H31N7O8S/c1-3-5-8-23-18-15-19(26-22(25-18)38-9-4-2)29(28-27-15)20-17(34)16(33)12(37-20)6-7-13(30)24-11(21(35)36)10-14(31)32/h6-7,11-12,16-17,20,33-34H,3-5,8-10H2,1-2H3,(H,24,30)(H,31,32)(H,35,36)(H,23,25,26)/b7-6+/t11-,12+,16+,17+,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y12 receptor assessed as ADP-induced human platelet aggregation |
Bioorg Med Chem Lett 17: 6013-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.057
BindingDB Entry DOI: 10.7270/Q2JD4Z2Q |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50594691
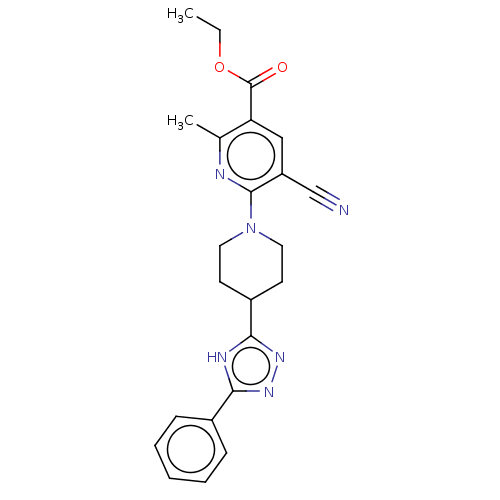
(CHEMBL5176593)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)c1nnc([nH]1)-c1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113924
BindingDB Entry DOI: 10.7270/Q2R215CH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50118225

(ARL 69931MX | Adenosine triphosphate derivative | ...)Show SMILES CSCCNc1nc(SCCC(F)(F)F)nc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C17H25Cl2F3N5O12P3S2/c1-43-5-3-23-12-9-13(26-15(25-12)44-4-2-16(20,21)22)27(7-24-9)14-11(29)10(28)8(38-14)6-37-42(35,36)39-41(33,34)17(18,19)40(30,31)32/h7-8,10-11,14,28-29H,2-6H2,1H3,(H,33,34)(H,35,36)(H,23,25,26)(H2,30,31,32)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Galecto Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation by turbidimetric method |
Bioorg Med Chem Lett 26: 2739-2754 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.030
BindingDB Entry DOI: 10.7270/Q2FF3V86 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50118225

(ARL 69931MX | Adenosine triphosphate derivative | ...)Show SMILES CSCCNc1nc(SCCC(F)(F)F)nc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C17H25Cl2F3N5O12P3S2/c1-43-5-3-23-12-9-13(26-15(25-12)44-4-2-16(20,21)22)27(7-24-9)14-11(29)10(28)8(38-14)6-37-42(35,36)39-41(33,34)17(18,19)40(30,31)32/h7-8,10-11,14,28-29H,2-6H2,1H3,(H,33,34)(H,35,36)(H,23,25,26)(H2,30,31,32)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes
Curated by ChEMBL
| Assay Description
The compound was evaluated for antagonist activity against platelet P2Y purinoceptor 12 (P2Y12) |
J Med Chem 45: 4057-93 (2002)
BindingDB Entry DOI: 10.7270/Q2VX0H71 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50118225

(ARL 69931MX | Adenosine triphosphate derivative | ...)Show SMILES CSCCNc1nc(SCCC(F)(F)F)nc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C17H25Cl2F3N5O12P3S2/c1-43-5-3-23-12-9-13(26-15(25-12)44-4-2-16(20,21)22)27(7-24-9)14-11(29)10(28)8(38-14)6-37-42(35,36)39-41(33,34)17(18,19)40(30,31)32/h7-8,10-11,14,28-29H,2-6H2,1H3,(H,33,34)(H,35,36)(H,23,25,26)(H2,30,31,32)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128837
BindingDB Entry DOI: 10.7270/Q2765K8C |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50423387
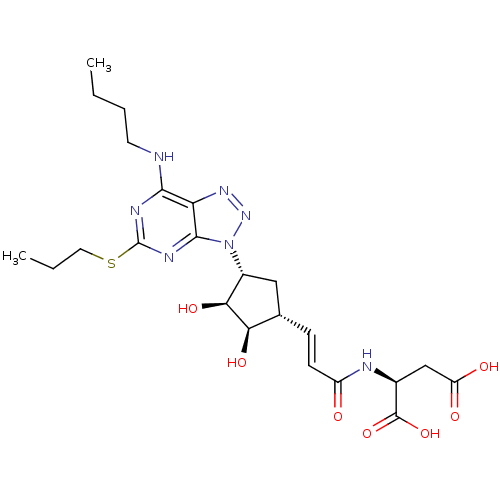
(CHEMBL437204)Show SMILES CCCCNc1nc(SCCC)nc2n(nnc12)[C@@H]1C[C@H](\C=C\C(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C23H33N7O7S/c1-3-5-8-24-20-17-21(27-23(26-20)38-9-4-2)30(29-28-17)14-10-12(18(34)19(14)35)6-7-15(31)25-13(22(36)37)11-16(32)33/h6-7,12-14,18-19,34-35H,3-5,8-11H2,1-2H3,(H,25,31)(H,32,33)(H,36,37)(H,24,26,27)/b7-6+/t12-,13-,14+,18+,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2Y12 receptor assessed as ADP-induced human platelet aggregation |
Bioorg Med Chem Lett 17: 6013-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.057
BindingDB Entry DOI: 10.7270/Q2JD4Z2Q |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50318031

(CHEMBL1097279 | cangrelor)Show SMILES CSCCNc1nc(SCCC(F)(F)F)nc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)C(Cl)(Cl)P([O-])([O-])=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H25Cl2F3N5O12P3S2/c1-43-5-3-23-12-9-13(26-15(25-12)44-4-2-16(20,21)22)27(7-24-9)14-11(29)10(28)8(38-14)6-37-42(35,36)39-41(33,34)17(18,19)40(30,31)32/h7-8,10-11,14,28-29H,2-6H2,1H3,(H,33,34)(H,35,36)(H,23,25,26)(H2,30,31,32)/p-4/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor by [35S]GTPgammaS binding assay |
J Med Chem 53: 3489-501 (2010)
Article DOI: 10.1021/jm901691y
BindingDB Entry DOI: 10.7270/Q28C9WFW |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50167270
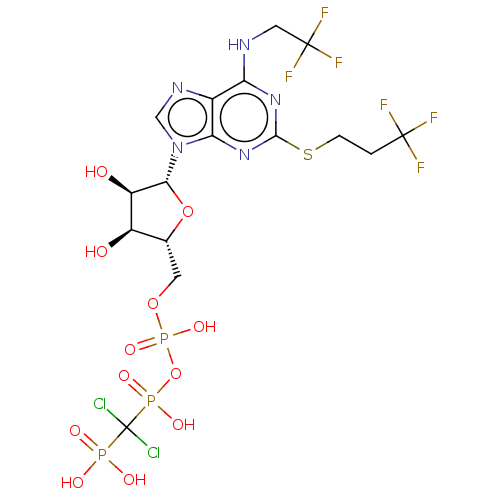
(CHEMBL1160364)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1COP(O)(=O)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)n1cnc2c(NCC(F)(F)F)nc(SCCC(F)(F)F)nc12 |r| Show InChI InChI=1S/C16H20Cl2F6N5O12P3S/c17-16(18,42(32,33)34)43(35,36)41-44(37,38)39-3-6-8(30)9(31)12(40-6)29-5-26-7-10(25-4-15(22,23)24)27-13(28-11(7)29)45-2-1-14(19,20)21/h5-6,8-9,12,30-31H,1-4H2,(H,35,36)(H,37,38)(H,25,27,28)(H2,32,33,34)/t6-,8-,9-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Galecto Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation by turbidimetric method |
Bioorg Med Chem Lett 26: 2739-2754 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.030
BindingDB Entry DOI: 10.7270/Q2FF3V86 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50100263
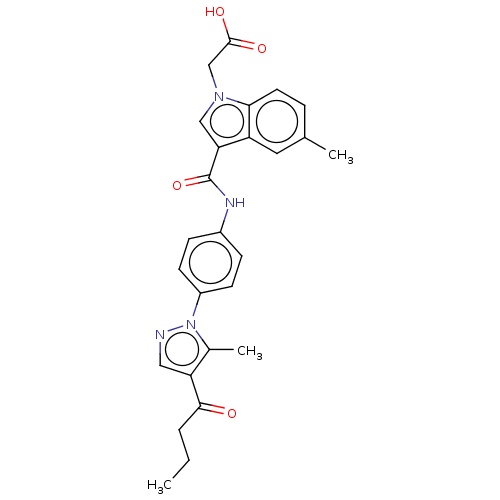
(CHEMBL3326907)Show SMILES CCCC(=O)c1cnn(c1C)-c1ccc(NC(=O)c2cn(CC(O)=O)c3ccc(C)cc23)cc1 Show InChI InChI=1S/C26H26N4O4/c1-4-5-24(31)21-13-27-30(17(21)3)19-9-7-18(8-10-19)28-26(34)22-14-29(15-25(32)33)23-11-6-16(2)12-20(22)23/h6-14H,4-5,15H2,1-3H3,(H,28,34)(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis |
J Med Chem 57: 7293-316 (2014)
Article DOI: 10.1021/jm500588w
BindingDB Entry DOI: 10.7270/Q2D79D21 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50594684
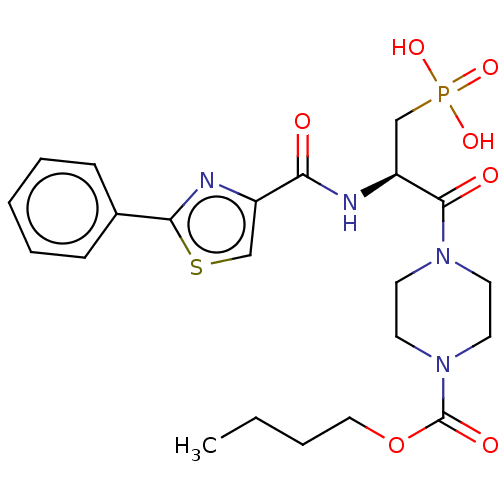
(CHEMBL5179719)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CP(O)(O)=O)NC(=O)c1csc(n1)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113924
BindingDB Entry DOI: 10.7270/Q2R215CH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50594685

(CHEMBL5184268)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1ccc(cc1)P(O)(O)=O)NC(=O)c1csc(n1)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113924
BindingDB Entry DOI: 10.7270/Q2R215CH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50594686
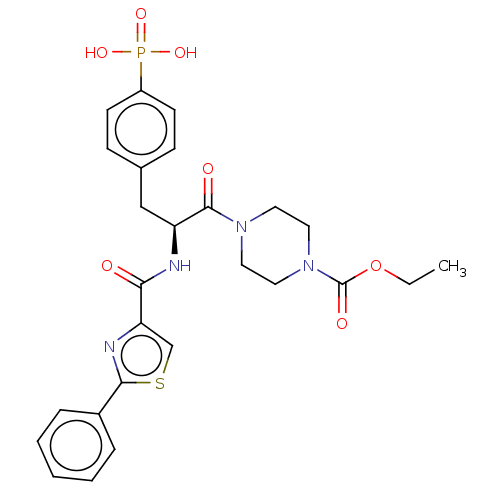
(CHEMBL5195237)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](Cc1ccc(cc1)P(O)(O)=O)NC(=O)c1csc(n1)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113924
BindingDB Entry DOI: 10.7270/Q2R215CH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017
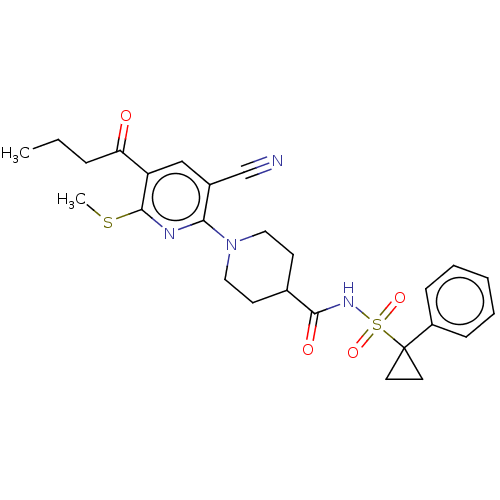
(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity P2Y12 receptor in human washed platelets assessed as inhibition of ADP-induced platelet aggregation after 5 to 90 mins by spectro... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50594688

(CHEMBL5192953)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CP(O)(O)=O)NC(=O)c1nc(sc1N1CCN(C)CC1)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113924
BindingDB Entry DOI: 10.7270/Q2R215CH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50594689

(CHEMBL5203416)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CP(O)(O)=O)NC(=O)c1nc(sc1N(C)CCCC)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113924
BindingDB Entry DOI: 10.7270/Q2R215CH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50594687

(CHEMBL5173489)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CCC(O)=O)NC(=O)c1nc(sc1N1CC[C@@H](C1)OC)-c1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113924
BindingDB Entry DOI: 10.7270/Q2R215CH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50167271
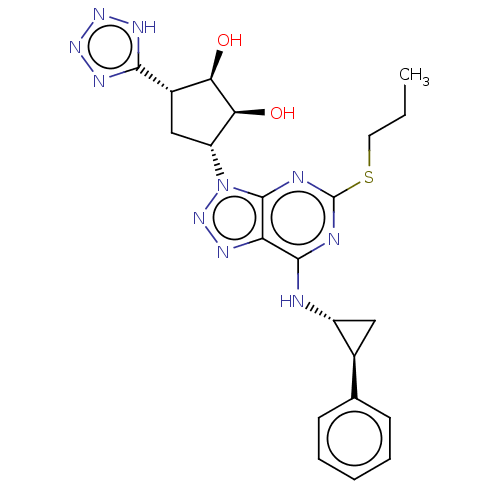
(CHEMBL3798413)Show SMILES CCCSc1nc(N[C@@H]2C[C@H]2c2ccccc2)c2nnn([C@@H]3C[C@@H]([C@@H](O)[C@H]3O)c3nnn[nH]3)c2n1 |r| Show InChI InChI=1S/C22H26N10O2S/c1-2-8-35-22-24-20(23-14-9-12(14)11-6-4-3-5-7-11)16-21(25-22)32(31-26-16)15-10-13(17(33)18(15)34)19-27-29-30-28-19/h3-7,12-15,17-18,33-34H,2,8-10H2,1H3,(H,23,24,25)(H,27,28,29,30)/t12-,13-,14+,15+,17+,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Galecto Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation by microplate reader analysis |
Bioorg Med Chem Lett 26: 2739-2754 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.030
BindingDB Entry DOI: 10.7270/Q2FF3V86 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50118225

(ARL 69931MX | Adenosine triphosphate derivative | ...)Show SMILES CSCCNc1nc(SCCC(F)(F)F)nc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C17H25Cl2F3N5O12P3S2/c1-43-5-3-23-12-9-13(26-15(25-12)44-4-2-16(20,21)22)27(7-24-9)14-11(29)10(28)8(38-14)6-37-42(35,36)39-41(33,34)17(18,19)40(30,31)32/h7-8,10-11,14,28-29H,2-6H2,1H3,(H,33,34)(H,35,36)(H,23,25,26)(H2,30,31,32)/t8-,10-,11-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of ADP-induced aggregation preincubated for 5 mins followed by... |
Bioorg Med Chem 23: 3880-906 (2015)
Article DOI: 10.1016/j.bmc.2014.12.034
BindingDB Entry DOI: 10.7270/Q2MC91S4 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50167271

(CHEMBL3798413)Show SMILES CCCSc1nc(N[C@@H]2C[C@H]2c2ccccc2)c2nnn([C@@H]3C[C@@H]([C@@H](O)[C@H]3O)c3nnn[nH]3)c2n1 |r| Show InChI InChI=1S/C22H26N10O2S/c1-2-8-35-22-24-20(23-14-9-12(14)11-6-4-3-5-7-11)16-21(25-22)32(31-26-16)15-10-13(17(33)18(15)34)19-27-29-30-28-19/h3-7,12-15,17-18,33-34H,2,8-10H2,1H3,(H,23,24,25)(H,27,28,29,30)/t12-,13-,14+,15+,17+,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Galecto Biotech
Curated by ChEMBL
| Assay Description
Displacement of [3H]-2-Mes-ADP from human P2Y12 receptor by beta-counter analysis |
Bioorg Med Chem Lett 26: 2739-2754 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.030
BindingDB Entry DOI: 10.7270/Q2FF3V86 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50118223
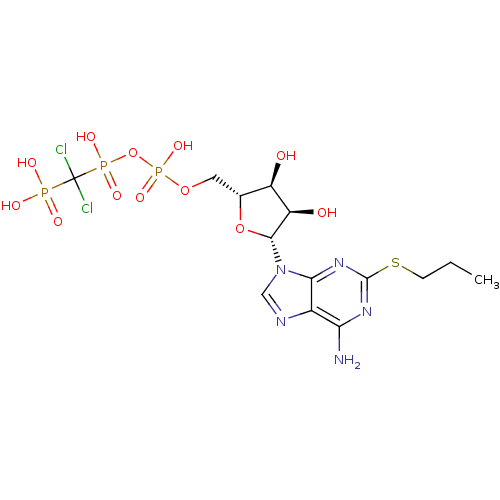
(AR-C67085 | Adenosine triphosphate derivative | CH...)Show SMILES CCCSc1nc(N)c2ncn([C@@H]3O[C@H](COP(O)(=O)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C14H22Cl2N5O12P3S/c1-2-3-37-13-19-10(17)7-11(20-13)21(5-18-7)12-9(23)8(22)6(32-12)4-31-36(29,30)33-35(27,28)14(15,16)34(24,25)26/h5-6,8-9,12,22-23H,2-4H2,1H3,(H,27,28)(H,29,30)(H2,17,19,20)(H2,24,25,26)/t6-,8-,9-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Galecto Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation by turbidimetric method |
Bioorg Med Chem Lett 26: 2739-2754 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.030
BindingDB Entry DOI: 10.7270/Q2FF3V86 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50594682
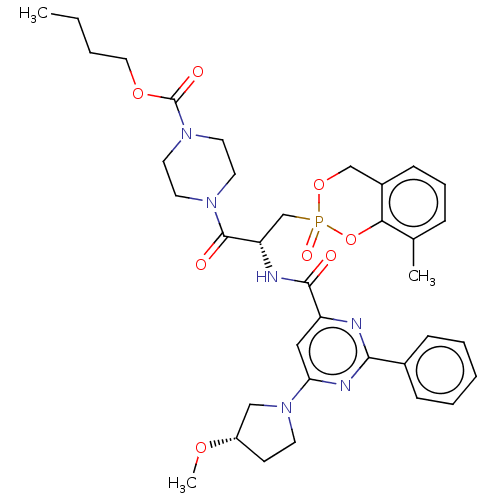
(CHEMBL5196387)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CP1(=O)OCc2cccc(C)c2O1)NC(=O)c1cc(nc(n1)-c1ccccc1)N1CC[C@@H](C1)OC |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113924
BindingDB Entry DOI: 10.7270/Q2R215CH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM119428
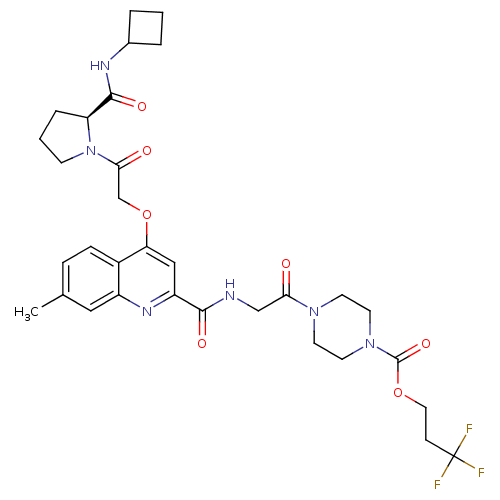
(US8669266, 679)Show SMILES Cc1ccc2c(OCC(=O)N3CCC[C@H]3C(=O)NC3CCC3)cc(nc2c1)C(=O)NCC(=O)N1CCN(CC1)C(=O)OCCC(F)(F)F Show InChI InChI=1S/C32H39F3N6O7/c1-20-7-8-22-23(16-20)38-24(17-26(22)48-19-28(43)41-10-3-6-25(41)30(45)37-21-4-2-5-21)29(44)36-18-27(42)39-11-13-40(14-12-39)31(46)47-15-9-32(33,34)35/h7-8,16-17,21,25H,2-6,9-15,18-19H2,1H3,(H,36,44)(H,37,45)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113924
BindingDB Entry DOI: 10.7270/Q2R215CH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50594681

(CHEMBL5188874)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CP(O)(O)=O)NC(=O)c1cc(nc(n1)-c1ccccc1)N1CCN(C)CC1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113924
BindingDB Entry DOI: 10.7270/Q2R215CH |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50057329

(CHEMBL3322653)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CCC(O)=O)NC(=O)c1cc(nc(n1)-c1ccccc1)[C@H]1C[C@@H]1COC |r| Show InChI InChI=1S/C30H39N5O7/c1-3-4-16-42-30(40)35-14-12-34(13-15-35)29(39)23(10-11-26(36)37)33-28(38)25-18-24(22-17-21(22)19-41-2)31-27(32-25)20-8-6-5-7-9-20/h5-9,18,21-23H,3-4,10-17,19H2,1-2H3,(H,33,38)(H,36,37)/t21-,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-2-methyl-thio-adenosine 5'-diphosphate from human recombinant P2Y12 expressed in CHO cell membranes by scintillation counting me... |
Bioorg Med Chem Lett 24: 4323-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.070
BindingDB Entry DOI: 10.7270/Q28C9XW4 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50057328

(CHEMBL3322652)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CCC(O)=O)NC(=O)c1cc(nc(n1)-c1ccccc1)N1CC[C@@H](C1)OC |r| Show InChI InChI=1S/C30H40N6O7/c1-3-4-18-43-30(41)35-16-14-34(15-17-35)29(40)23(10-11-26(37)38)32-28(39)24-19-25(36-13-12-22(20-36)42-2)33-27(31-24)21-8-6-5-7-9-21/h5-9,19,22-23H,3-4,10-18,20H2,1-2H3,(H,32,39)(H,37,38)/t22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-2-methyl-thio-adenosine 5'-diphosphate from human recombinant P2Y12 expressed in CHO cell membranes by scintillation counting me... |
Bioorg Med Chem Lett 24: 4323-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.070
BindingDB Entry DOI: 10.7270/Q28C9XW4 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50057329

(CHEMBL3322653)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CCC(O)=O)NC(=O)c1cc(nc(n1)-c1ccccc1)[C@H]1C[C@@H]1COC |r| Show InChI InChI=1S/C30H39N5O7/c1-3-4-16-42-30(40)35-14-12-34(13-15-35)29(39)23(10-11-26(36)37)33-28(38)25-18-24(22-17-21(22)19-41-2)31-27(32-25)20-8-6-5-7-9-20/h5-9,18,21-23H,3-4,10-17,19H2,1-2H3,(H,33,38)(H,36,37)/t21-,22+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-2-methyl-thio-adenosine 5'-diphosphate from human recombinant P2Y12 expressed in CHO cell membranes by scintillation counting me... |
Bioorg Med Chem Lett 24: 4323-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.070
BindingDB Entry DOI: 10.7270/Q28C9XW4 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM119428

(US8669266, 679)Show SMILES Cc1ccc2c(OCC(=O)N3CCC[C@H]3C(=O)NC3CCC3)cc(nc2c1)C(=O)NCC(=O)N1CCN(CC1)C(=O)OCCC(F)(F)F Show InChI InChI=1S/C32H39F3N6O7/c1-20-7-8-22-23(16-20)38-24(17-26(22)48-19-28(43)41-10-3-6-25(41)30(45)37-21-4-2-5-21)29(44)36-18-27(42)39-11-13-40(14-12-39)31(46)47-15-9-32(33,34)35/h7-8,16-17,21,25H,2-6,9-15,18-19H2,1H3,(H,36,44)(H,37,45)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
The ability of a test compound to bind to the P2Y12 receptor was evaluated in a recombinant cell membrane binding assay. In this competitive binding ... |
US Patent US8669266 (2014)
BindingDB Entry DOI: 10.7270/Q2DJ5D9R |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM119426

(US8669266, 354)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CCO)NC(=O)c1cc(OCC(=O)N2CCC[C@H]2C(=O)NC2CCC2)c2ccc(C)cc2n1 Show InChI InChI=1S/C35H48N6O8/c1-3-4-19-48-35(47)40-16-14-39(15-17-40)34(46)26(12-18-42)38-32(44)28-21-30(25-11-10-23(2)20-27(25)37-28)49-22-31(43)41-13-6-9-29(41)33(45)36-24-7-5-8-24/h10-11,20-21,24,26,29,42H,3-9,12-19,22H2,1-2H3,(H,36,45)(H,38,44)/t26-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
The ability of a test compound to bind to the P2Y12 receptor was evaluated in a recombinant cell membrane binding assay. In this competitive binding ... |
US Patent US8669266 (2014)
BindingDB Entry DOI: 10.7270/Q2DJ5D9R |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017

(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 60 mins |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM119431
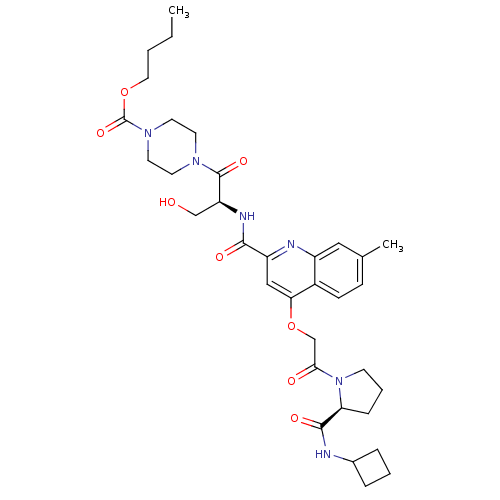
(US8669266, 528)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CO)NC(=O)c1cc(OCC(=O)N2CCC[C@H]2C(=O)NC2CCC2)c2ccc(C)cc2n1 Show InChI InChI=1S/C34H46N6O8/c1-3-4-17-47-34(46)39-15-13-38(14-16-39)33(45)27(20-41)37-31(43)26-19-29(24-11-10-22(2)18-25(24)36-26)48-21-30(42)40-12-6-9-28(40)32(44)35-23-7-5-8-23/h10-11,18-19,23,27-28,41H,3-9,12-17,20-21H2,1-2H3,(H,35,44)(H,37,43)/t27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
The ability of a test compound to bind to the P2Y12 receptor was evaluated in a recombinant cell membrane binding assay. In this competitive binding ... |
US Patent US8669266 (2014)
BindingDB Entry DOI: 10.7270/Q2DJ5D9R |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50397205
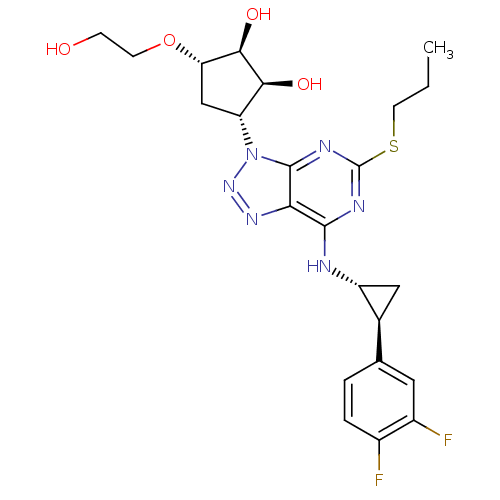
(AR-C126532XX | AZD-6140 | AZD6140 | BRILINTA | TIC...)Show SMILES CCCSc1nc(N[C@@H]2C[C@H]2c2ccc(F)c(F)c2)c2nnn([C@@H]3C[C@H](OCCO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C23H28F2N6O4S/c1-2-7-36-23-27-21(26-15-9-12(15)11-3-4-13(24)14(25)8-11)18-22(28-23)31(30-29-18)16-10-17(35-6-5-32)20(34)19(16)33/h3-4,8,12,15-17,19-20,32-34H,2,5-7,9-10H2,1H3,(H,26,27,28)/t12-,15+,16+,17-,19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Galecto Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor (unknown origin) |
Bioorg Med Chem Lett 26: 2739-2754 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.030
BindingDB Entry DOI: 10.7270/Q2FF3V86 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM119427

(US8669266, 490)Show SMILES CCCOC(=O)N1CCN(CC1)C(=O)[C@H](CCC(=O)OC)NC(=O)c1cc(OCC(=O)N2CCC[C@H]2C(=O)NC2CCC2)c2ccc(C)cc2n1 Show InChI InChI=1S/C36H48N6O9/c1-4-19-50-36(48)41-17-15-40(16-18-41)35(47)26(12-13-32(44)49-3)39-33(45)28-21-30(25-11-10-23(2)20-27(25)38-28)51-22-31(43)42-14-6-9-29(42)34(46)37-24-7-5-8-24/h10-11,20-21,24,26,29H,4-9,12-19,22H2,1-3H3,(H,37,46)(H,39,45)/t26-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
The ability of a test compound to bind to the P2Y12 receptor was evaluated in a recombinant cell membrane binding assay. In this competitive binding ... |
US Patent US8669266 (2014)
BindingDB Entry DOI: 10.7270/Q2DJ5D9R |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM119429
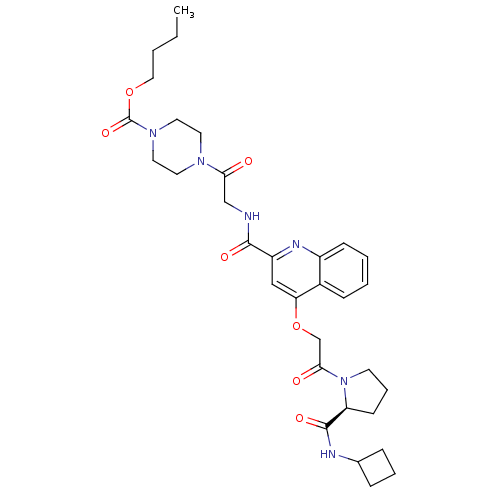
(US8669266, 673)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)CNC(=O)c1cc(OCC(=O)N2CCC[C@H]2C(=O)NC2CCC2)c2ccccc2n1 Show InChI InChI=1S/C32H42N6O7/c1-2-3-18-44-32(43)37-16-14-36(15-17-37)28(39)20-33-30(41)25-19-27(23-10-4-5-11-24(23)35-25)45-21-29(40)38-13-7-12-26(38)31(42)34-22-8-6-9-22/h4-5,10-11,19,22,26H,2-3,6-9,12-18,20-21H2,1H3,(H,33,41)(H,34,42)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
The ability of a test compound to bind to the P2Y12 receptor was evaluated in a recombinant cell membrane binding assay. In this competitive binding ... |
US Patent US8669266 (2014)
BindingDB Entry DOI: 10.7270/Q2DJ5D9R |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM119424
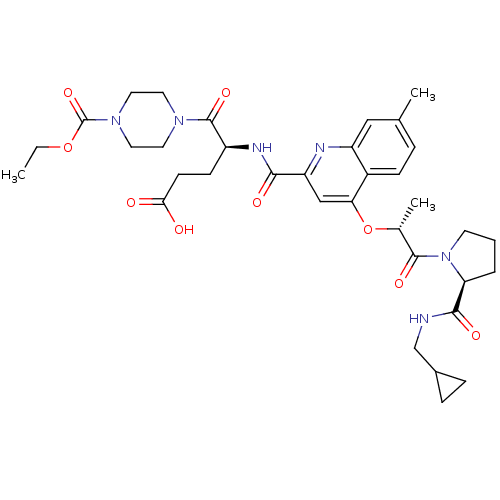
(US8669266, 300)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](CCC(O)=O)NC(=O)c1cc(O[C@H](C)C(=O)N2CCC[C@H]2C(=O)NCC2CC2)c2ccc(C)cc2n1 Show InChI InChI=1S/C35H46N6O9/c1-4-49-35(48)40-16-14-39(15-17-40)34(47)25(11-12-30(42)43)38-31(44)27-19-29(24-10-7-21(2)18-26(24)37-27)50-22(3)33(46)41-13-5-6-28(41)32(45)36-20-23-8-9-23/h7,10,18-19,22-23,25,28H,4-6,8-9,11-17,20H2,1-3H3,(H,36,45)(H,38,44)(H,42,43)/t22-,25+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
The ability of a test compound to bind to the P2Y12 receptor was evaluated in a recombinant cell membrane binding assay. In this competitive binding ... |
US Patent US8669266 (2014)
BindingDB Entry DOI: 10.7270/Q2DJ5D9R |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50100269
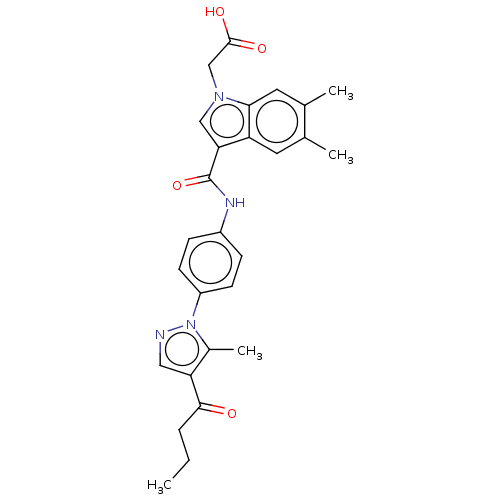
(CHEMBL3325627)Show SMILES CCCC(=O)c1cnn(c1C)-c1ccc(NC(=O)c2cn(CC(O)=O)c3cc(C)c(C)cc23)cc1 Show InChI InChI=1S/C27H28N4O4/c1-5-6-25(32)22-13-28-31(18(22)4)20-9-7-19(8-10-20)29-27(35)23-14-30(15-26(33)34)24-12-17(3)16(2)11-21(23)24/h7-14H,5-6,15H2,1-4H3,(H,29,35)(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis |
J Med Chem 57: 7293-316 (2014)
Article DOI: 10.1021/jm500588w
BindingDB Entry DOI: 10.7270/Q2D79D21 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50167273
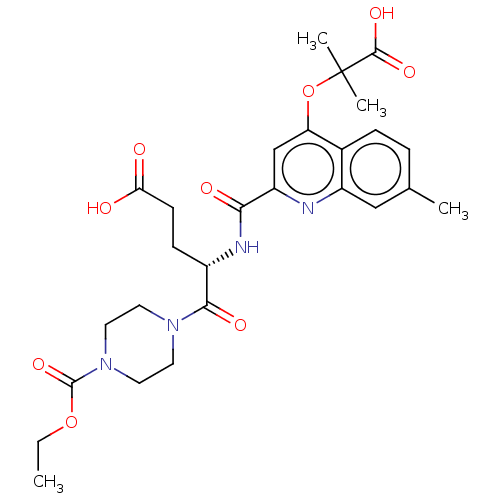
(CHEMBL3797316)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](CCC(O)=O)NC(=O)c1cc(OC(C)(C)C(O)=O)c2ccc(C)cc2n1 |r| Show InChI InChI=1S/C27H34N4O9/c1-5-39-26(38)31-12-10-30(11-13-31)24(35)18(8-9-22(32)33)29-23(34)20-15-21(40-27(3,4)25(36)37)17-7-6-16(2)14-19(17)28-20/h6-7,14-15,18H,5,8-13H2,1-4H3,(H,29,34)(H,32,33)(H,36,37)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Galecto Biotech
Curated by ChEMBL
| Assay Description
Displacement of [3H]-2-Mes-ADP from human P2Y12 receptor |
Bioorg Med Chem Lett 26: 2739-2754 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.030
BindingDB Entry DOI: 10.7270/Q2FF3V86 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436963
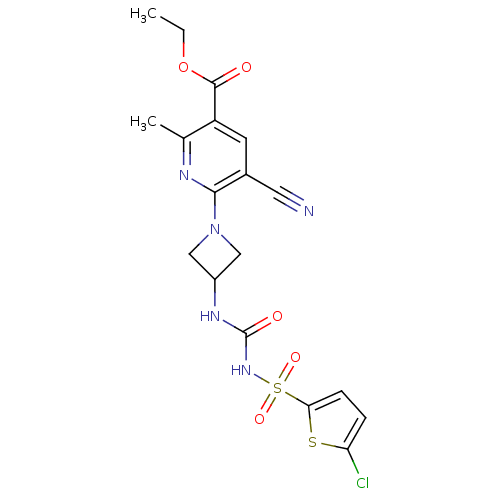
(CHEMBL2402255)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C18H18ClN5O5S2/c1-3-29-17(25)13-6-11(7-20)16(21-10(13)2)24-8-12(9-24)22-18(26)23-31(27,28)15-5-4-14(19)30-15/h4-6,12H,3,8-9H2,1-2H3,(H2,22,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50439278
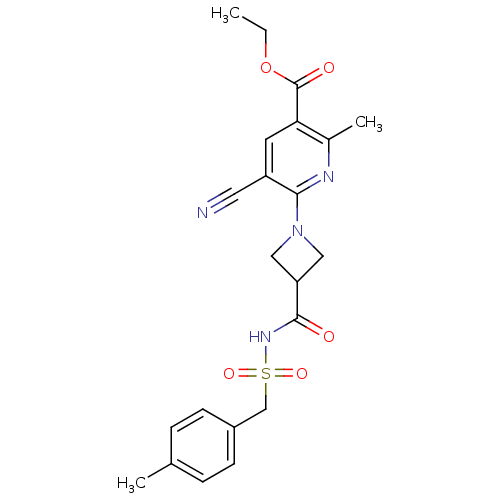
(CHEMBL2419487)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)C(=O)NS(=O)(=O)Cc1ccc(C)cc1 Show InChI InChI=1S/C22H24N4O5S/c1-4-31-22(28)19-9-17(10-23)20(24-15(19)3)26-11-18(12-26)21(27)25-32(29,30)13-16-7-5-14(2)6-8-16/h5-9,18H,4,11-13H2,1-3H3,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) expressed in CHO cell membrane after 1 hr by scintillation counting analysis |
J Med Chem 56: 7015-24 (2013)
Article DOI: 10.1021/jm400820m
BindingDB Entry DOI: 10.7270/Q29K4CNV |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50436963

(CHEMBL2402255)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CC(C1)NC(=O)NS(=O)(=O)c1ccc(Cl)s1 Show InChI InChI=1S/C18H18ClN5O5S2/c1-3-29-17(25)13-6-11(7-20)16(21-10(13)2)24-8-12(9-24)22-18(26)23-31(27,28)15-5-4-14(19)30-15/h4-6,12H,3,8-9H2,1-2H3,(H2,22,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) after 1 hr by scintillation counting analysis |
Eur J Med Chem 65: 360-75 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.007
BindingDB Entry DOI: 10.7270/Q2PK0HJ2 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50100195
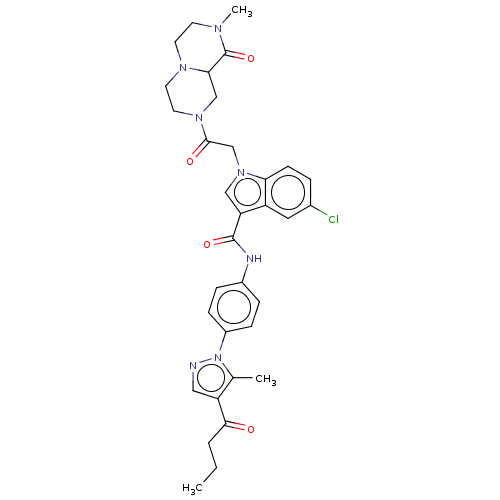
(CHEMBL3325805)Show SMILES CCCC(=O)c1cnn(c1C)-c1ccc(NC(=O)c2cn(CC(=O)N3CCN4CCN(C)C(=O)C4C3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C33H36ClN7O4/c1-4-5-30(42)26-17-35-41(21(26)2)24-9-7-23(8-10-24)36-32(44)27-18-40(28-11-6-22(34)16-25(27)28)20-31(43)39-15-14-38-13-12-37(3)33(45)29(38)19-39/h6-11,16-18,29H,4-5,12-15,19-20H2,1-3H3,(H,36,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation |
J Med Chem 57: 7293-316 (2014)
Article DOI: 10.1021/jm500588w
BindingDB Entry DOI: 10.7270/Q2D79D21 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50100195

(CHEMBL3325805)Show SMILES CCCC(=O)c1cnn(c1C)-c1ccc(NC(=O)c2cn(CC(=O)N3CCN4CCN(C)C(=O)C4C3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C33H36ClN7O4/c1-4-5-30(42)26-17-35-41(21(26)2)24-9-7-23(8-10-24)36-32(44)27-18-40(28-11-6-22(34)16-25(27)28)20-31(43)39-15-14-38-13-12-37(3)33(45)29(38)19-39/h6-11,16-18,29H,4-5,12-15,19-20H2,1-3H3,(H,36,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation |
J Med Chem 57: 7293-316 (2014)
Article DOI: 10.1021/jm500588w
BindingDB Entry DOI: 10.7270/Q2D79D21 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
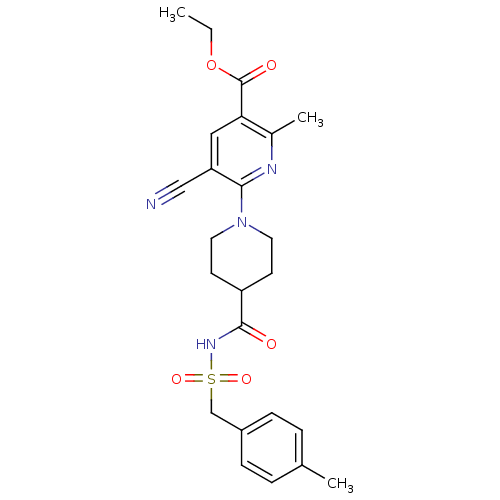
(Homo sapiens (Human)) | BDBM50439274
(CHEMBL2419493)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccc(C)cc1 Show InChI InChI=1S/C24H28N4O5S/c1-4-33-24(30)21-13-20(14-25)22(26-17(21)3)28-11-9-19(10-12-28)23(29)27-34(31,32)15-18-7-5-16(2)6-8-18/h5-8,13,19H,4,9-12,15H2,1-3H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]-AZ11931285 from P2Y12 receptor (unknown origin) expressed in CHO cell membrane after 1 hr by scintillation counting analysis |
J Med Chem 56: 7015-24 (2013)
Article DOI: 10.1021/jm400820m
BindingDB Entry DOI: 10.7270/Q29K4CNV |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50100262

(CHEMBL3326906)Show SMILES CCCC(=O)c1cnn(c1C)-c1ccc(NC(=O)c2cn(CCC(O)=O)c3ccc(C)cc23)cc1 Show InChI InChI=1S/C27H28N4O4/c1-4-5-25(32)22-15-28-31(18(22)3)20-9-7-19(8-10-20)29-27(35)23-16-30(13-12-26(33)34)24-11-6-17(2)14-21(23)24/h6-11,14-16H,4-5,12-13H2,1-3H3,(H,29,35)(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis |
J Med Chem 57: 7293-316 (2014)
Article DOI: 10.1021/jm500588w
BindingDB Entry DOI: 10.7270/Q2D79D21 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
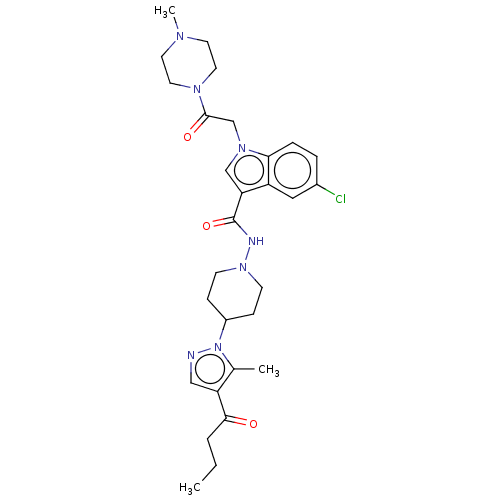
(Homo sapiens (Human)) | BDBM50100245
(CHEMBL3325894)Show SMILES CCCC(=O)c1cnn(C2CCN(CC2)NC(=O)c2cn(CC(=O)N3CCN(C)CC3)c3ccc(Cl)cc23)c1C Show InChI InChI=1S/C29H38ClN7O3/c1-4-5-27(38)24-17-31-37(20(24)2)22-8-10-36(11-9-22)32-29(40)25-18-35(26-7-6-21(30)16-23(25)26)19-28(39)34-14-12-33(3)13-15-34/h6-7,16-18,22H,4-5,8-15,19H2,1-3H3,(H,32,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis |
J Med Chem 57: 7293-316 (2014)
Article DOI: 10.1021/jm500588w
BindingDB Entry DOI: 10.7270/Q2D79D21 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50100195

(CHEMBL3325805)Show SMILES CCCC(=O)c1cnn(c1C)-c1ccc(NC(=O)c2cn(CC(=O)N3CCN4CCN(C)C(=O)C4C3)c3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C33H36ClN7O4/c1-4-5-30(42)26-17-35-41(21(26)2)24-9-7-23(8-10-24)36-32(44)27-18-40(28-11-6-22(34)16-25(27)28)20-31(43)39-15-14-38-13-12-37(3)33(45)29(38)19-39/h6-11,16-18,29H,4-5,12-15,19-20H2,1-3H3,(H,36,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in Sprague-Dawley rat platelet rich plasma assessed as inhibition of ADP-induced aggregation |
J Med Chem 57: 7293-316 (2014)
Article DOI: 10.1021/jm500588w
BindingDB Entry DOI: 10.7270/Q2D79D21 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM119430

(US8669266, 600)Show SMILES CCCCOC(=O)N1CCN(CC1)C(=O)[C@H](CCc1nnn[nH]1)NC(=O)c1cc(OCC(=O)N2CCC[C@H]2C(=O)NC2CCC2)c2ccc(C)cc2n1 Show InChI InChI=1S/C36H48N10O7/c1-3-4-19-52-36(51)45-17-15-44(16-18-45)35(50)26(12-13-31-40-42-43-41-31)39-33(48)28-21-30(25-11-10-23(2)20-27(25)38-28)53-22-32(47)46-14-6-9-29(46)34(49)37-24-7-5-8-24/h10-11,20-21,24,26,29H,3-9,12-19,22H2,1-2H3,(H,37,49)(H,39,48)(H,40,41,42,43)/t26-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
The ability of a test compound to bind to the P2Y12 receptor was evaluated in a recombinant cell membrane binding assay. In this competitive binding ... |
US Patent US8669266 (2014)
BindingDB Entry DOI: 10.7270/Q2DJ5D9R |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50167273

(CHEMBL3797316)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](CCC(O)=O)NC(=O)c1cc(OC(C)(C)C(O)=O)c2ccc(C)cc2n1 |r| Show InChI InChI=1S/C27H34N4O9/c1-5-39-26(38)31-12-10-30(11-13-31)24(35)18(8-9-22(32)33)29-23(34)20-15-21(40-27(3,4)25(36)37)17-7-6-16(2)14-19(17)28-20/h6-7,14-15,18H,5,8-13H2,1-4H3,(H,29,34)(H,32,33)(H,36,37)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Galecto Biotech
Curated by ChEMBL
| Assay Description
Antagonist activity at P2Y12 receptor in human washed platelets assessed as inhibition of ADP-induced platelet aggregation measured for 5 mins by lig... |
Bioorg Med Chem Lett 26: 2739-2754 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.030
BindingDB Entry DOI: 10.7270/Q2FF3V86 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM119422

(US8669266, 270)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)[C@H](CC(O)=O)NC(=O)c1cc(OCC(=O)N2CCC[C@H]2C(=O)NC2CCC2)c2cc(F)c(C)cc2n1 Show InChI InChI=1S/C33H41FN6O9/c1-3-48-33(47)39-12-10-38(11-13-39)32(46)25(17-29(42)43)37-30(44)24-16-27(21-15-22(34)19(2)14-23(21)36-24)49-18-28(41)40-9-5-8-26(40)31(45)35-20-6-4-7-20/h14-16,20,25-26H,3-13,17-18H2,1-2H3,(H,35,45)(H,37,44)(H,42,43)/t25-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
US Patent
| Assay Description
The ability of a test compound to bind to the P2Y12 receptor was evaluated in a recombinant cell membrane binding assay. In this competitive binding ... |
US Patent US8669266 (2014)
BindingDB Entry DOI: 10.7270/Q2DJ5D9R |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
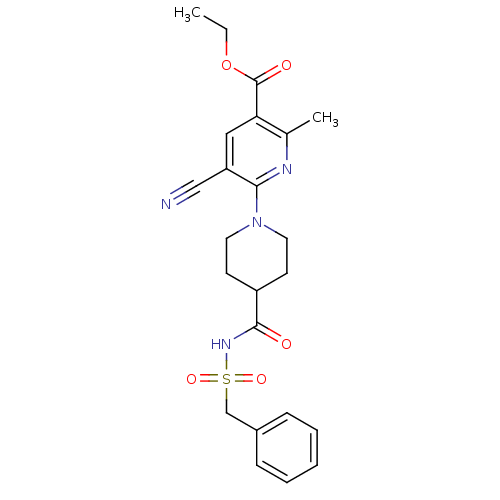
(Homo sapiens (Human)) | BDBM50439277
(CHEMBL2419490)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H26N4O5S/c1-3-32-23(29)20-13-19(14-24)21(25-16(20)2)27-11-9-18(10-12-27)22(28)26-33(30,31)15-17-7-5-4-6-8-17/h4-8,13,18H,3,9-12,15H2,1-2H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 60 mins |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50439277

(CHEMBL2419490)Show SMILES CCOC(=O)c1cc(C#N)c(nc1C)N1CCC(CC1)C(=O)NS(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C23H26N4O5S/c1-3-32-23(29)20-13-19(14-24)21(25-16(20)2)27-11-9-18(10-12-27)22(28)26-33(30,31)15-17-7-5-4-6-8-17/h4-8,13,18H,3,9-12,15H2,1-2H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity P2Y12 receptor in human washed platelets assessed as inhibition of ADP-induced platelet aggregation after 5 to 90 mins by spectro... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data