

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50368140 (CHEMBL3706401 | CHEMBL611854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition constants with Met (L-methionine) substrate site of M-2 (kidney form) variant of rat Methionine adenosyltransferase | J Med Chem 33: 2545-51 (1990) BindingDB Entry DOI: 10.7270/Q2SJ1M6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50368140 (CHEMBL3706401 | CHEMBL611854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition constants against ATP substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase | J Med Chem 33: 2545-51 (1990) BindingDB Entry DOI: 10.7270/Q2SJ1M6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50227269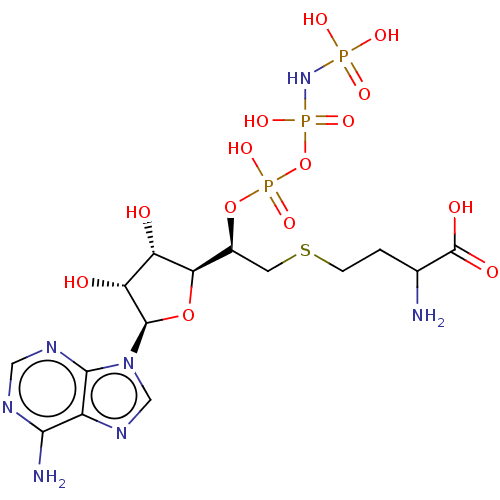 (CHEMBL3706402) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition constants against ATP substrate site of M-2 (kidney form) variant of rat Methionine adenosyltransferase | J Med Chem 33: 2545-51 (1990) BindingDB Entry DOI: 10.7270/Q2SJ1M6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50227269 (CHEMBL3706402) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition constants against Met (L-methionine)P substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase | J Med Chem 33: 2545-51 (1990) BindingDB Entry DOI: 10.7270/Q2SJ1M6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50227269 (CHEMBL3706402) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition constants with Met (L-methionine) substrate site of M-2 (kidney form) variant of rat Methionine adenosyltransferase | J Med Chem 33: 2545-51 (1990) BindingDB Entry DOI: 10.7270/Q2SJ1M6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50227269 (CHEMBL3706402) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition constants against Met (L-methionine)P substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase | J Med Chem 33: 2545-51 (1990) BindingDB Entry DOI: 10.7270/Q2SJ1M6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50368140 (CHEMBL3706401 | CHEMBL611854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition constants against Met (L-methionine)P substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase | J Med Chem 33: 2545-51 (1990) BindingDB Entry DOI: 10.7270/Q2SJ1M6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50368140 (CHEMBL3706401 | CHEMBL611854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition constants against ATP substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase | J Med Chem 33: 2545-51 (1990) BindingDB Entry DOI: 10.7270/Q2SJ1M6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50367305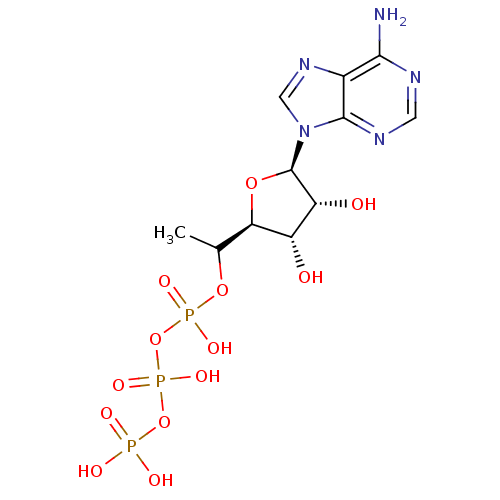 (CHEMBL1791430) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat liver Methionine adenosyltransferase I, activity expressed as Ki | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50367303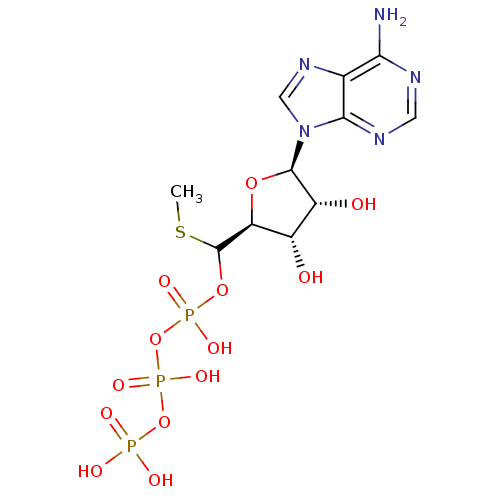 (CHEMBL1791433) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat liver Methionine adenosyltransferase I, activity expressed as Ki | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50367042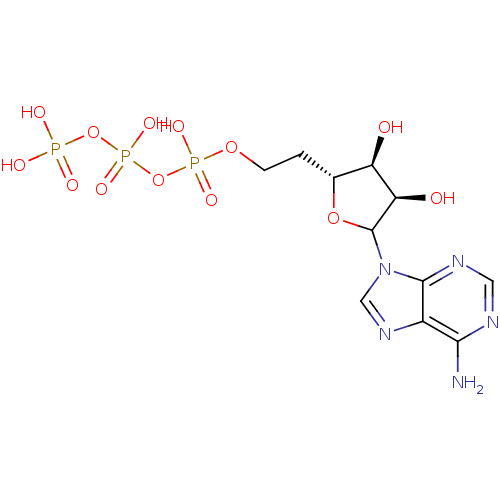 (CHEMBL606221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat liver Methionine adenosyltransferase I | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50070637 (CHEMBL295971 | L-2-amino-4-methoxy-cis-but-3-enoic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem | MMDB PDB PubMed | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U.M.R. 6519 Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liver | Bioorg Med Chem Lett 8: 1629-34 (1999) BindingDB Entry DOI: 10.7270/Q2QR4W8K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50368141 (CHEMBL3706400 | CHEMBL610658) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition constants against ATP substrate site of M-2 (kidney form) variant of rat Methionine adenosyltransferase | J Med Chem 33: 2545-51 (1990) BindingDB Entry DOI: 10.7270/Q2SJ1M6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50368141 (CHEMBL3706400 | CHEMBL610658) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition constants against ATP substrate site of M-T (Novikoff Ascitic Hepatoma form) variant of rat Methionine adenosyltransferase | J Med Chem 33: 2545-51 (1990) BindingDB Entry DOI: 10.7270/Q2SJ1M6S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50070638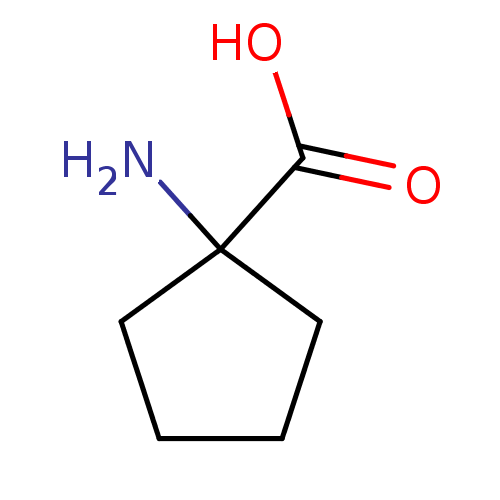 (1-AMINOCYCLOPENTANECARBOXYLIC ACID | 1-Amino-cyclo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 5.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U.M.R. 6519 Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liver | Bioorg Med Chem Lett 8: 1629-34 (1999) BindingDB Entry DOI: 10.7270/Q2QR4W8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50070635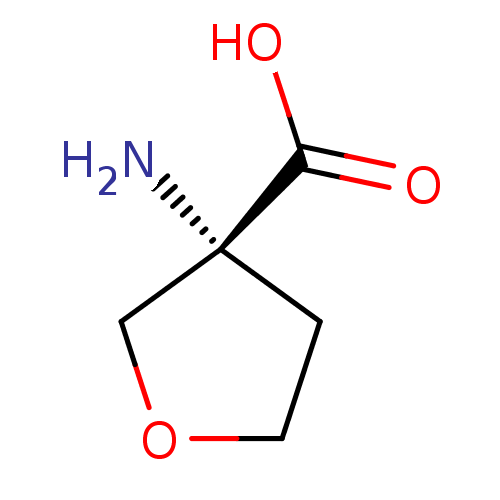 ((R)-3-Amino-tetrahydro-furan-3-carboxylic acid | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U.M.R. 6519 Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liver | Bioorg Med Chem Lett 8: 1629-34 (1999) BindingDB Entry DOI: 10.7270/Q2QR4W8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50070634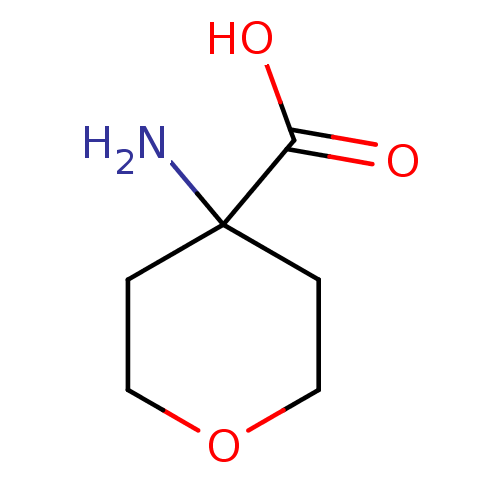 (4-Amino-tetrahydro-pyran-4-carboxylic acid | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U.M.R. 6519 Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liver | Bioorg Med Chem Lett 8: 1629-34 (1999) BindingDB Entry DOI: 10.7270/Q2QR4W8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50070636 ((S)-3-Amino-tetrahydro-thiophene-3-carboxylic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 1.04E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U.M.R. 6519 Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liver | Bioorg Med Chem Lett 8: 1629-34 (1999) BindingDB Entry DOI: 10.7270/Q2QR4W8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50070639 (4-Amino-tetrahydro-thiopyran-4-carboxylic acid | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
U.M.R. 6519 Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liver | Bioorg Med Chem Lett 8: 1629-34 (1999) BindingDB Entry DOI: 10.7270/Q2QR4W8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine synthase isoform type-1 (Rattus norvegicus) | BDBM50367306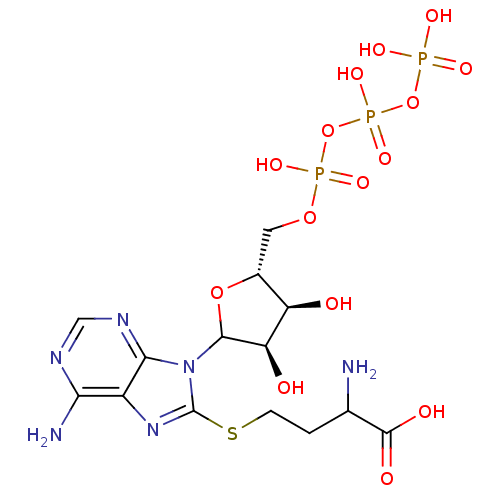 (CHEMBL608929) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat liver Methionine adenosyltransferase I | J Med Chem 29: 318-22 (1986) BindingDB Entry DOI: 10.7270/Q2DF6RSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||