 Found 14 hits of ki for UniProtKB: P97687
Found 14 hits of ki for UniProtKB: P97687 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50378649
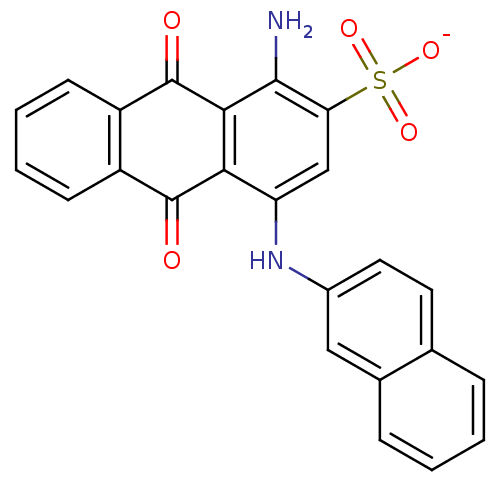
(CHEMBL597197)Show SMILES Nc1c2C(=O)c3ccccc3C(=O)c2c(Nc2ccc3ccccc3c2)cc1S([O-])(=O)=O Show InChI InChI=1S/C24H16N2O5S/c25-22-19(32(29,30)31)12-18(26-15-10-9-13-5-1-2-6-14(13)11-15)20-21(22)24(28)17-8-4-3-7-16(17)23(20)27/h1-12,26H,25H2,(H,29,30,31)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 328 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat CD39 expressed in CHO cells using ATP as substrate incubated for 10 mins by UV absorbance method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50227019
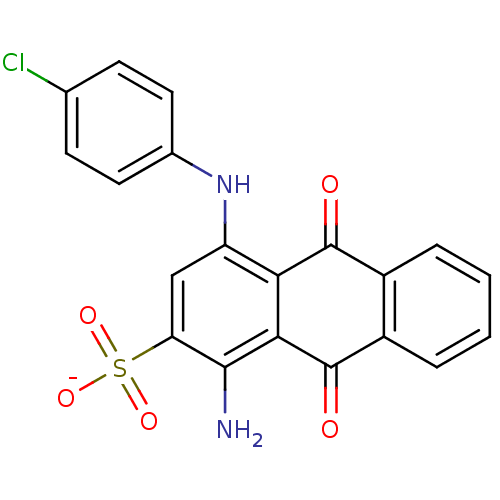
(CHEMBL271673 | sodium 1-amino-4-(4-chlorophenylami...)Show SMILES Nc1c(cc(Nc2ccc(Cl)cc2)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C20H13ClN2O5S/c21-10-5-7-11(8-6-10)23-14-9-15(29(26,27)28)18(22)17-16(14)19(24)12-3-1-2-4-13(12)20(17)25/h1-9,23H,22H2,(H,26,27,28)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat CD39 expressed in CHO cells using ATP as substrate incubated for 10 mins by UV absorbance method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50029031
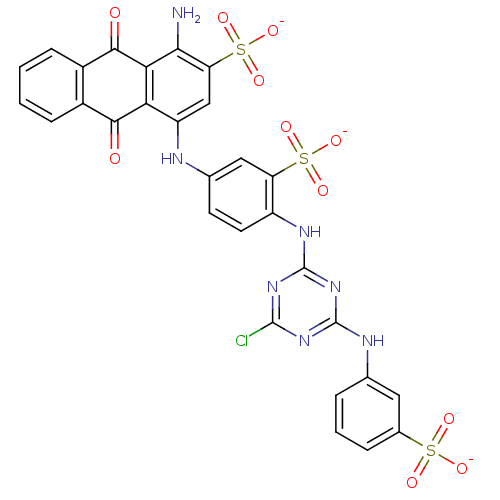
(1-Amino-4-{4-[4-chloro-6-(3-sulfo-phenylamino)-[1,...)Show SMILES Nc1c(cc(Nc2ccc(Nc3nc(Cl)nc(Nc4cccc(c4)S([O-])(=O)=O)n3)c(c2)S([O-])(=O)=O)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C29H20ClN7O11S3/c30-27-35-28(33-13-4-3-5-15(10-13)49(40,41)42)37-29(36-27)34-18-9-8-14(11-20(18)50(43,44)45)32-19-12-21(51(46,47)48)24(31)23-22(19)25(38)16-6-1-2-7-17(16)26(23)39/h1-12,32H,31H2,(H,40,41,42)(H,43,44,45)(H,46,47,48)(H2,33,34,35,36,37)/p-3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant NTPDase1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5943-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.003
BindingDB Entry DOI: 10.7270/Q27H1KC9 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50029031

(1-Amino-4-{4-[4-chloro-6-(3-sulfo-phenylamino)-[1,...)Show SMILES Nc1c(cc(Nc2ccc(Nc3nc(Cl)nc(Nc4cccc(c4)S([O-])(=O)=O)n3)c(c2)S([O-])(=O)=O)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C29H20ClN7O11S3/c30-27-35-28(33-13-4-3-5-15(10-13)49(40,41)42)37-29(36-27)34-18-9-8-14(11-20(18)50(43,44)45)32-19-12-21(51(46,47)48)24(31)23-22(19)25(38)16-6-1-2-7-17(16)26(23)39/h1-12,32H,31H2,(H,40,41,42)(H,43,44,45)(H,46,47,48)(H2,33,34,35,36,37)/p-3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPdase1 by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50029031

(1-Amino-4-{4-[4-chloro-6-(3-sulfo-phenylamino)-[1,...)Show SMILES Nc1c(cc(Nc2ccc(Nc3nc(Cl)nc(Nc4cccc(c4)S([O-])(=O)=O)n3)c(c2)S([O-])(=O)=O)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C29H20ClN7O11S3/c30-27-35-28(33-13-4-3-5-15(10-13)49(40,41)42)37-29(36-27)34-18-9-8-14(11-20(18)50(43,44)45)32-19-12-21(51(46,47)48)24(31)23-22(19)25(38)16-6-1-2-7-17(16)26(23)39/h1-12,32H,31H2,(H,40,41,42)(H,43,44,45)(H,46,47,48)(H2,33,34,35,36,37)/p-3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase 1 |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50552129
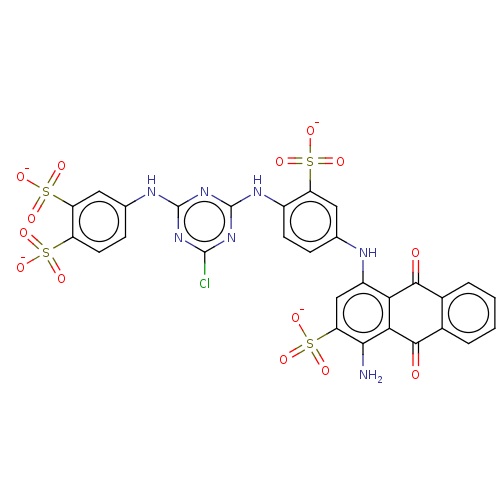
(CHEMBL4744335)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1c(cc(-[#7]-c2ccc(-[#7]-c3nc(Cl)nc(-[#7]-c4ccc(c(c4)S([#8-])(=O)=O)S([#8-])(=O)=O)n3)c(c2)S([#8-])(=O)=O)c2-[#6](=O)-c3ccccc3-[#6](=O)-c12)S([#8-])(=O)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat CD39 expressed in CHO cells using ATP as substrate incubated for 10 mins by UV absorbance method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01044
BindingDB Entry DOI: 10.7270/Q2B27ZZ6 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50195359
(ARL-67156 | CHEMBL223145)Show SMILES CCN(CC)c1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)C(Br)(Br)P([O-])([O-])=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H24Br2N5O12P3/c1-3-21(4-2)12-9-13(19-6-18-12)22(7-20-9)14-11(24)10(23)8(33-14)5-32-37(30,31)34-36(28,29)15(16,17)35(25,26)27/h6-8,10-11,14,23-24H,3-5H2,1-2H3,(H,28,29)(H,30,31)(H2,25,26,27)/p-4/t8-,10-,11-,14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant NTPDase1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5943-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.003
BindingDB Entry DOI: 10.7270/Q27H1KC9 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50195359

(ARL-67156 | CHEMBL223145)Show SMILES CCN(CC)c1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)C(Br)(Br)P([O-])([O-])=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H24Br2N5O12P3/c1-3-21(4-2)12-9-13(19-6-18-12)22(7-20-9)14-11(24)10(23)8(33-14)5-32-37(30,31)34-36(28,29)15(16,17)35(25,26)27/h6-8,10-11,14,23-24H,3-5H2,1-2H3,(H,28,29)(H,30,31)(H2,25,26,27)/p-4/t8-,10-,11-,14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase 1 |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50262422
(CHEMBL477339 | sodium (E)-4-((3-formyl-4-hydroxy-5...)Show SMILES Cc1cc(N=Nc2ccc(cc2S([O-])(=O)=O)S([O-])(=O)=O)c(COP([O-])([O-])=O)c(C=O)c1O |w:4.3| Show InChI InChI=1S/C15H15N2O12PS2/c1-8-4-13(11(7-29-30(20,21)22)10(6-18)15(8)19)17-16-12-3-2-9(31(23,24)25)5-14(12)32(26,27)28/h2-6,19H,7H2,1H3,(H2,20,21,22)(H,23,24,25)(H,26,27,28)/p-4 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase 1 |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50227032
(CHEMBL256057 | acid blue 25 | sodium 1-amino-9,10-...)Show SMILES Nc1c2C(=O)c3ccccc3C(=O)c2c(Nc2ccccc2)cc1S([O-])(=O)=O Show InChI InChI=1S/C20H14N2O5S/c21-18-15(28(25,26)27)10-14(22-11-6-2-1-3-7-11)16-17(18)20(24)13-9-5-4-8-12(13)19(16)23/h1-10,22H,21H2,(H,25,26,27)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPdase1 by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50336767
(CHEMBL257495 | PSB-716 | sodium 1-amino-4-(2-metho...)Show SMILES COc1ccccc1Nc1cc(c(N)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O Show InChI InChI=1S/C21H16N2O6S/c1-29-15-9-5-4-8-13(15)23-14-10-16(30(26,27)28)19(22)18-17(14)20(24)11-6-2-3-7-12(11)21(18)25/h2-10,23H,22H2,1H3,(H,26,27,28)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase 1 |
Bioorg Med Chem Lett 18: 223-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.082
BindingDB Entry DOI: 10.7270/Q2RX9CXT |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50268574
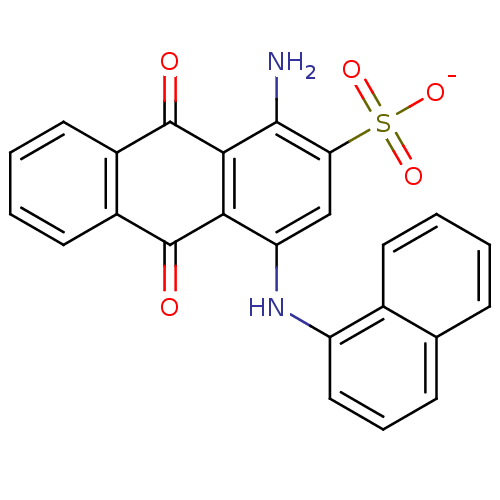
(CHEMBL498423 | Sodium 1-Amino-4-(1-naphthylamino)-...)Show SMILES Nc1c2C(=O)c3ccccc3C(=O)c2c(Nc2cccc3ccccc23)cc1S([O-])(=O)=O Show InChI InChI=1S/C24H16N2O5S/c25-22-19(32(29,30)31)12-18(26-17-11-5-7-13-6-1-2-8-14(13)17)20-21(22)24(28)16-10-4-3-9-15(16)23(20)27/h1-12,26H,25H2,(H,29,30,31)/p-1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPdase1 by capillary electrophoresis method |
J Med Chem 53: 2076-86 (2010)
Article DOI: 10.1021/jm901851t
BindingDB Entry DOI: 10.7270/Q2DZ097V |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50336799
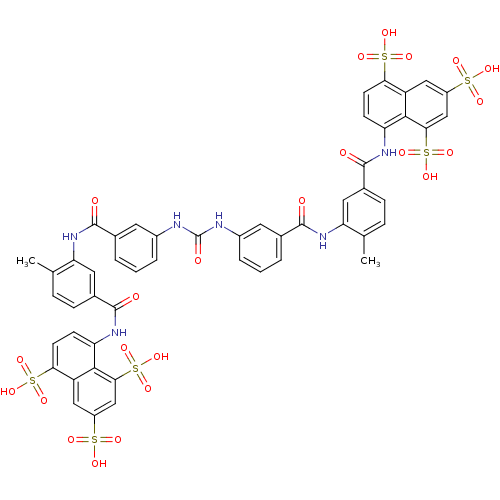
(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant NTPDase1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5943-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.003
BindingDB Entry DOI: 10.7270/Q27H1KC9 |
More data for this
Ligand-Target Pair | |
Ectonucleoside triphosphate diphosphohydrolase 1
(Rattus norvegicus) | BDBM50336799

(5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...)Show SMILES Cc1ccc(cc1NC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(=O)Nc2cc(ccc2C)C(=O)Nc2ccc(c3cc(cc(c23)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)c1)C(=O)Nc1ccc(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-24H,1-2H3,(H,54,60)(H,55,61)(H,56,58)(H,57,59)(H2,52,53,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of rat NTPDase 1 |
J Med Chem 51: 4518-28 (2008)
Article DOI: 10.1021/jm800175e
BindingDB Entry DOI: 10.7270/Q2XW4KQX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data