

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Mus musculus) | BDBM50352081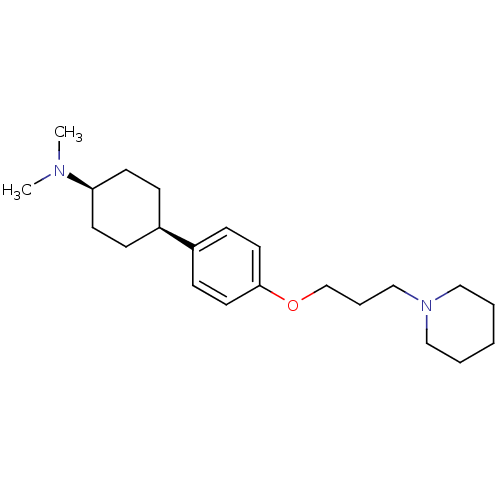 (CHEMBL1824230) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from mouse brain cortex histamine H3 receptor after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50352095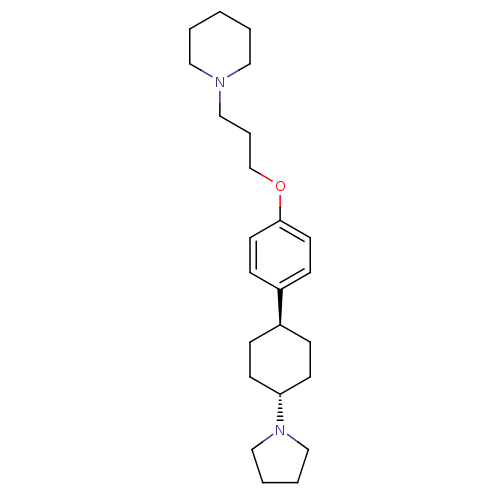 (CHEMBL1824235) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from mouse brain cortex histamine H3 receptor after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50352099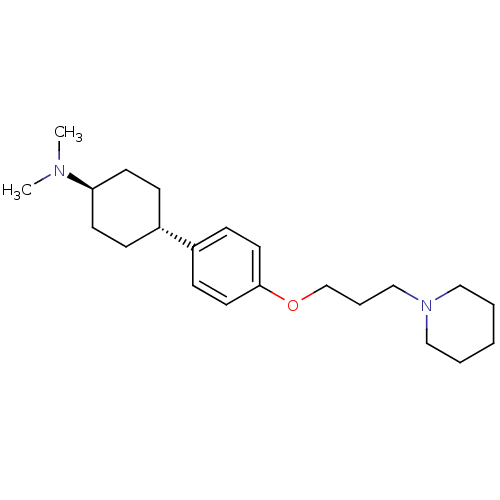 (CHEMBL1824231) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from mouse brain cortex histamine H3 receptor after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM22541 (Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of 3-([1,1,1-3H]methyl)-2-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-quinazolinone from mouse histamine H3 receptor expressed in HE... | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50514105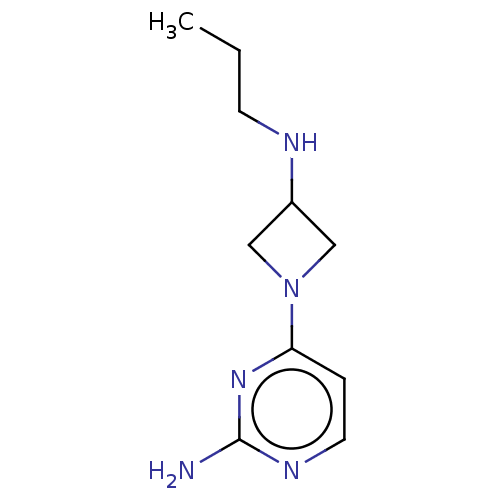 (CHEMBL4441731) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]NAMH from mouse H3R expressed in HEK293T cells incubated for 2 hrs by microbeta scintillation counting analysis | J Med Chem 62: 10848-10866 (2019) Article DOI: 10.1021/acs.jmedchem.9b01462 BindingDB Entry DOI: 10.7270/Q2M61PKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50352101 (CHEMBL1824236) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from mouse brain cortex histamine H3 receptor after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50352096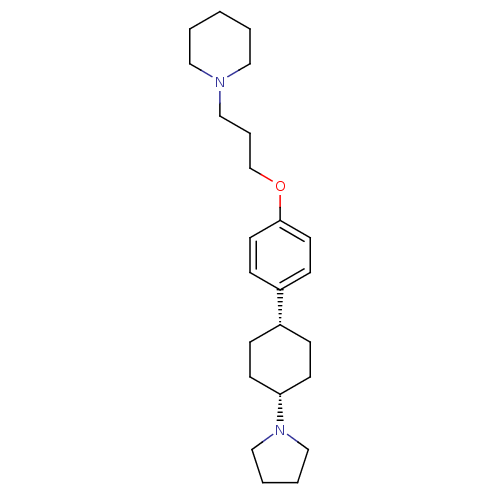 (CHEMBL1824234) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from mouse brain cortex histamine H3 receptor after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50352086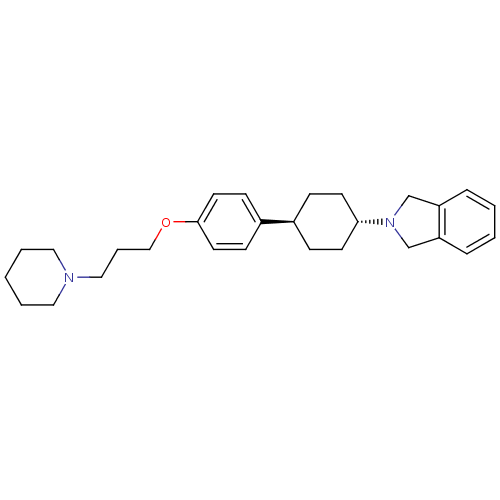 (CHEMBL1824245) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from mouse brain cortex histamine H3 receptor after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50352097 (CHEMBL1824233) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from mouse brain cortex histamine H3 receptor after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50352090 (CHEMBL1824241) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]-iodoproxyfan from histamine H3 receptor in mouse brain cortex after 1 hr by gamma counting analysis | Bioorg Med Chem Lett 23: 2548-54 (2013) Article DOI: 10.1016/j.bmcl.2013.02.118 BindingDB Entry DOI: 10.7270/Q2416ZFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50352090 (CHEMBL1824241) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from mouse brain cortex histamine H3 receptor after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50264895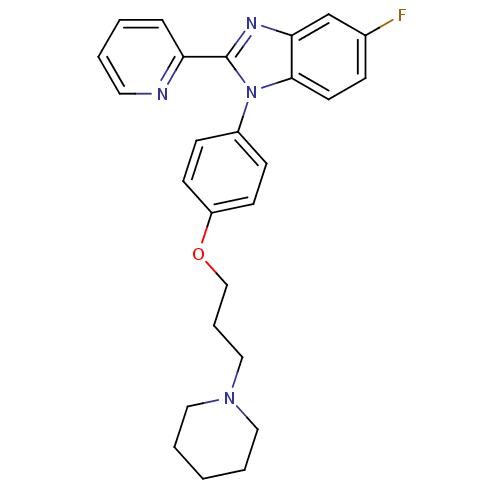 (5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity to mouse histamine H3 receptor | Bioorg Med Chem Lett 18: 5032-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.008 BindingDB Entry DOI: 10.7270/Q2CZ370Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441503 (CHEMBL2436628) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM22911 (2-(3H-imidazol-4-yl)ethylsulfanylmethanimidamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of 3-([1,1,1-3H]methyl)-2-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-quinazolinone from mouse histamine H3 receptor expressed in HE... | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441511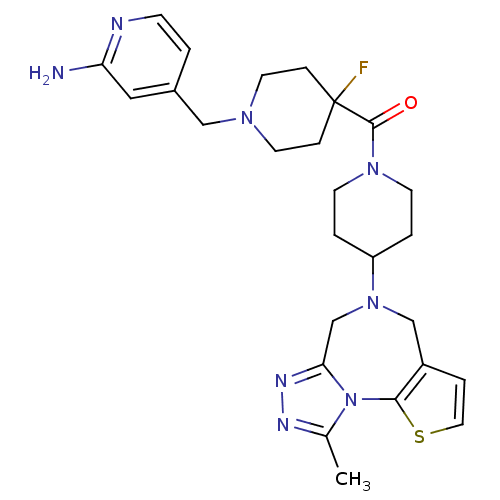 (CHEMBL2436620) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50352084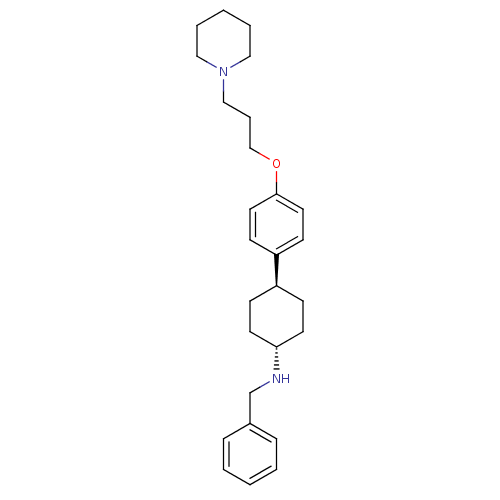 (CHEMBL1824247) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from mouse brain cortex histamine H3 receptor after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50352092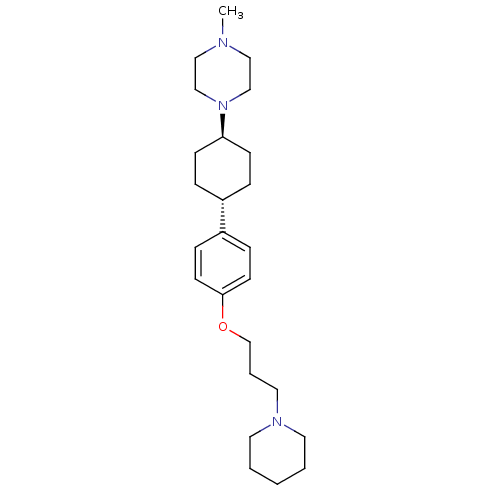 (CHEMBL1824239) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]Iodoproxyfan from mouse brain cortex histamine H3 receptor after 60 mins | Bioorg Med Chem Lett 21: 5384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.06.102 BindingDB Entry DOI: 10.7270/Q2DV1K7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50383442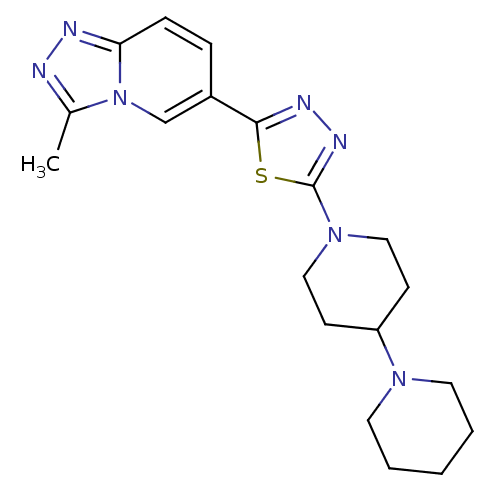 (CHEMBL2031602) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor | Bioorg Med Chem Lett 22: 3354-7 (2012) Article DOI: 10.1016/j.bmcl.2012.02.076 BindingDB Entry DOI: 10.7270/Q2DN462S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50246289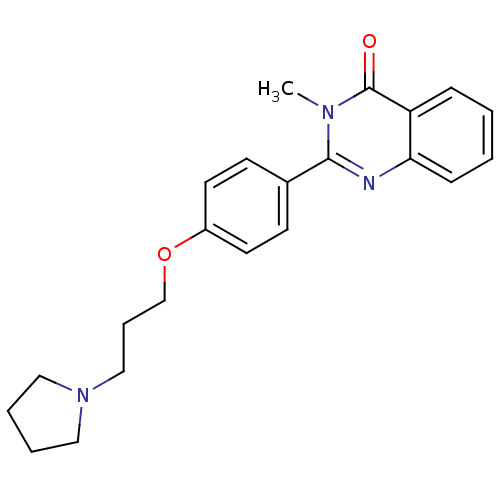 (3-methyl-2-(4-(3-(pyrrolidin-1-yl)propoxy)phenyl)q...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of 3-([1,1,1-3H]methyl)-2-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-quinazolinone from mouse histamine H3 receptor expressed in HE... | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50433200 (CHEMBL2375594) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]-iodoproxyfan from histamine H3 receptor in mouse brain cortex after 1 hr by gamma counting analysis | Bioorg Med Chem Lett 23: 2548-54 (2013) Article DOI: 10.1016/j.bmcl.2013.02.118 BindingDB Entry DOI: 10.7270/Q2416ZFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50383459 (CHEMBL2031619) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor | Bioorg Med Chem Lett 22: 3354-7 (2012) Article DOI: 10.1016/j.bmcl.2012.02.076 BindingDB Entry DOI: 10.7270/Q2DN462S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441506 (CHEMBL2436625) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441505 (CHEMBL2436626) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441509 (CHEMBL2436622) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274235 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274200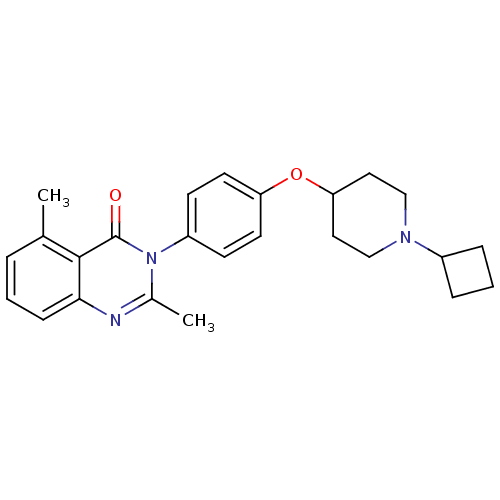 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-2,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441507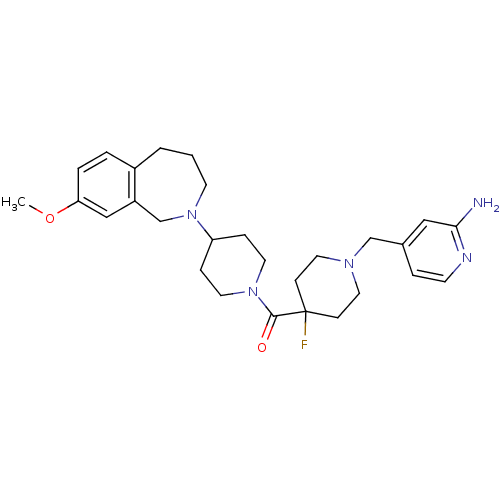 (CHEMBL2436624) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441514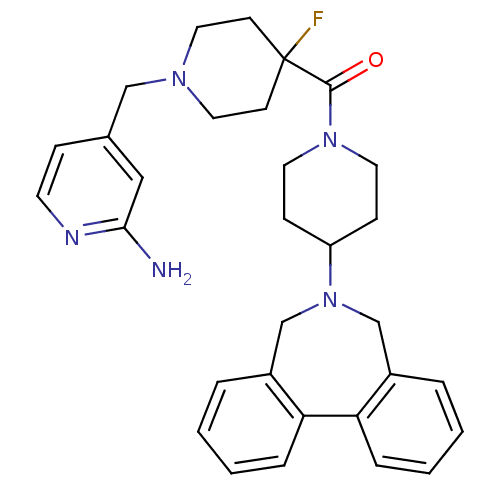 (CHEMBL2436633) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441502 (CHEMBL2436629) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386346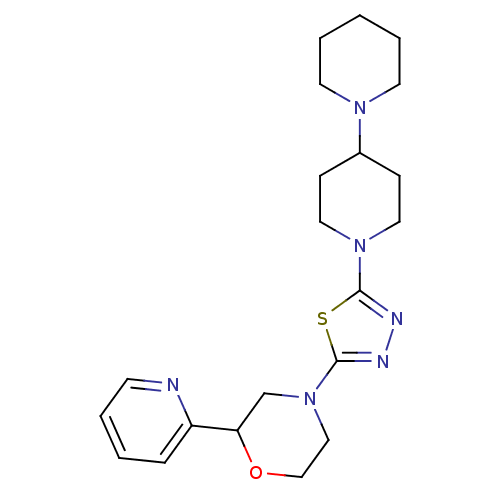 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441504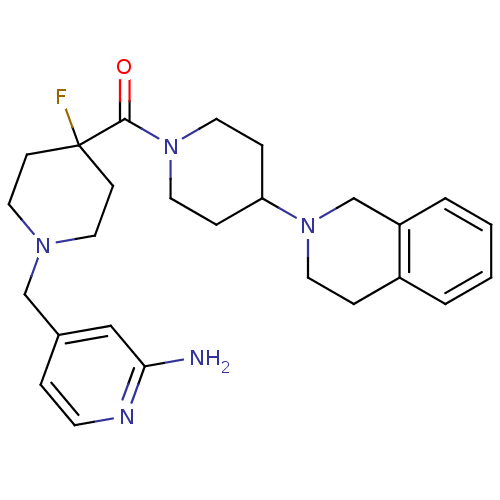 (CHEMBL2436627) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386346 (CHEMBL2048594) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50433199 (CHEMBL2375595) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]-iodoproxyfan from histamine H3 receptor in mouse brain cortex after 1 hr by gamma counting analysis | Bioorg Med Chem Lett 23: 2548-54 (2013) Article DOI: 10.1016/j.bmcl.2013.02.118 BindingDB Entry DOI: 10.7270/Q2416ZFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM22914 (CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of 3-([1,1,1-3H]methyl)-2-(4-{[3-(1-pyrrolidinyl)propyl]oxy}phenyl)-4(3H)-quinazolinone from mouse histamine H3 receptor expressed in HE... | Bioorg Med Chem Lett 19: 4075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.025 BindingDB Entry DOI: 10.7270/Q2GQ6XSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386351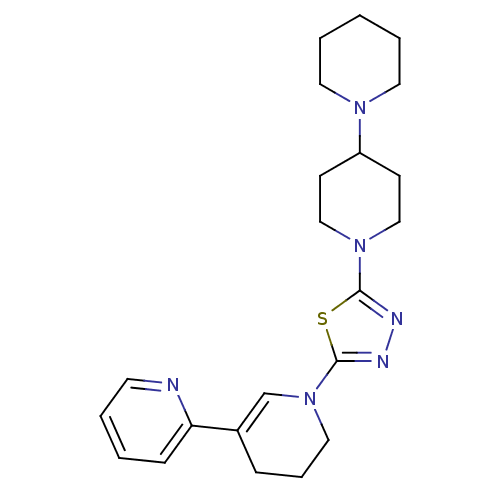 (CHEMBL2048589) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from mouse histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50433198 (CHEMBL2375588) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]-iodoproxyfan from histamine H3 receptor in mouse brain cortex after 1 hr by gamma counting analysis | Bioorg Med Chem Lett 23: 2548-54 (2013) Article DOI: 10.1016/j.bmcl.2013.02.118 BindingDB Entry DOI: 10.7270/Q2416ZFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50433194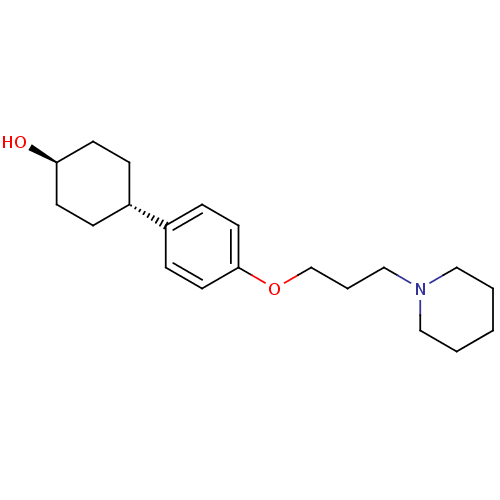 (CHEMBL2375587) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]-iodoproxyfan from histamine H3 receptor in mouse brain cortex after 1 hr by gamma counting analysis | Bioorg Med Chem Lett 23: 2548-54 (2013) Article DOI: 10.1016/j.bmcl.2013.02.118 BindingDB Entry DOI: 10.7270/Q2416ZFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441512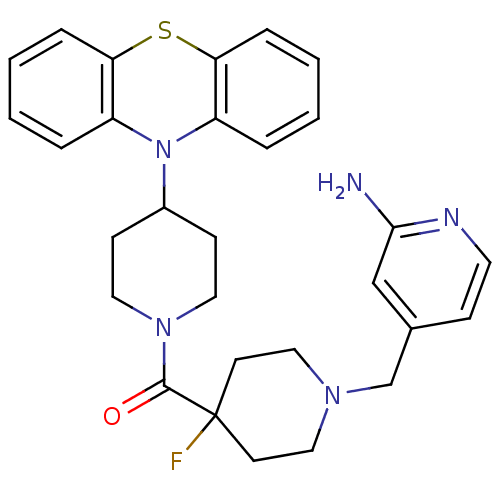 (CHEMBL2436617) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441501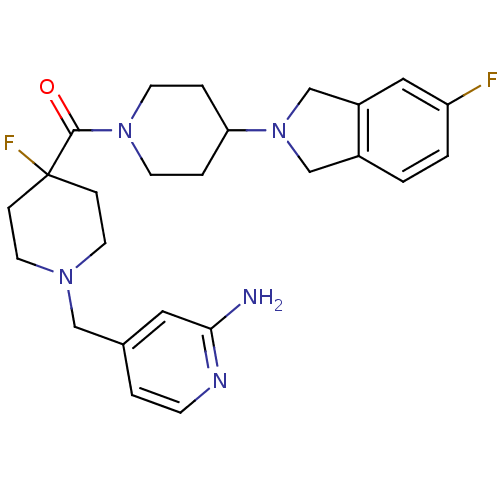 (CHEMBL2436630) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386364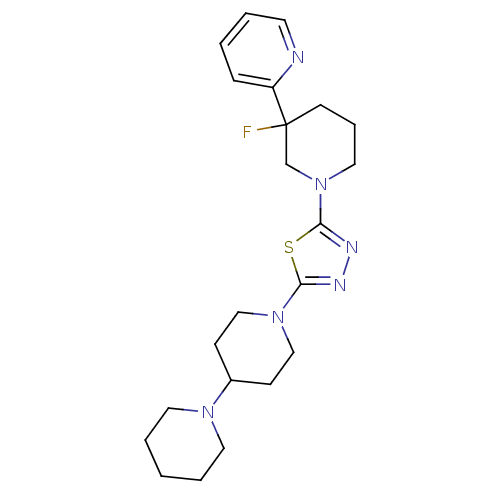 (CHEMBL2048592) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50383445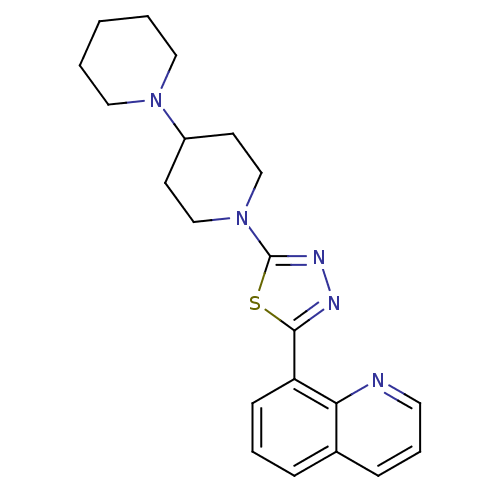 (CHEMBL2031605) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor | Bioorg Med Chem Lett 22: 3354-7 (2012) Article DOI: 10.1016/j.bmcl.2012.02.076 BindingDB Entry DOI: 10.7270/Q2DN462S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50433197 (CHEMBL2375597) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]-iodoproxyfan from histamine H3 receptor in mouse brain cortex after 1 hr by gamma counting analysis | Bioorg Med Chem Lett 23: 2548-54 (2013) Article DOI: 10.1016/j.bmcl.2013.02.118 BindingDB Entry DOI: 10.7270/Q2416ZFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50274692 (3-(4-[(1-Cyclobutyl-4-piperidinyl)oxy]phenyl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse recombinant histamine H3 receptor | J Med Chem 51: 6889-901 (2008) Article DOI: 10.1021/jm800569w BindingDB Entry DOI: 10.7270/Q27P8Z60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50383456 (CHEMBL2031616) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor | Bioorg Med Chem Lett 22: 3354-7 (2012) Article DOI: 10.1016/j.bmcl.2012.02.076 BindingDB Entry DOI: 10.7270/Q2DN462S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50433196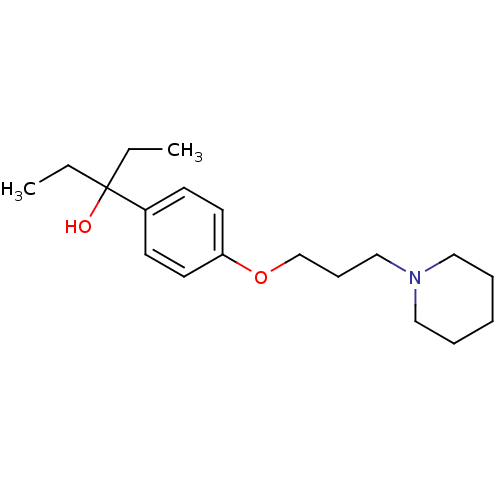 (CHEMBL2375593) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bioprojet-Biotech Curated by ChEMBL | Assay Description Displacement of [125I]-iodoproxyfan from histamine H3 receptor in mouse brain cortex after 1 hr by gamma counting analysis | Bioorg Med Chem Lett 23: 2548-54 (2013) Article DOI: 10.1016/j.bmcl.2013.02.118 BindingDB Entry DOI: 10.7270/Q2416ZFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [125I]-iodoproxyfan from mouse brain cortex H3 receptor after 60 mins by gamma counting method | Bioorg Med Chem 26: 2573-2585 (2018) Article DOI: 10.1016/j.bmc.2018.04.023 BindingDB Entry DOI: 10.7270/Q2TQ641P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50441517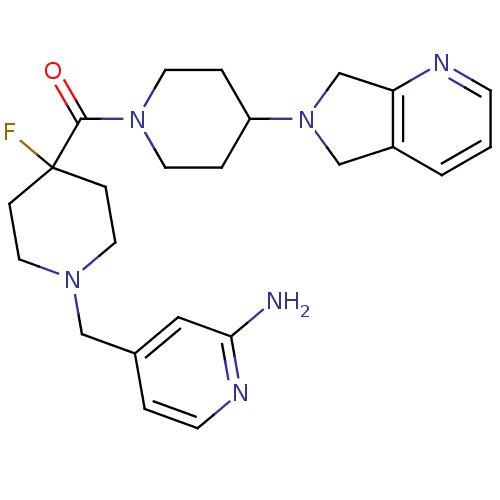 (CHEMBL2436631) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-alpha-methylhistamine from mouse histamine H3 receptor after 30 mins by scintillation counting analysis | Bioorg Med Chem Lett 23: 6004-9 (2013) Article DOI: 10.1016/j.bmcl.2013.08.013 BindingDB Entry DOI: 10.7270/Q2FJ2J67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386347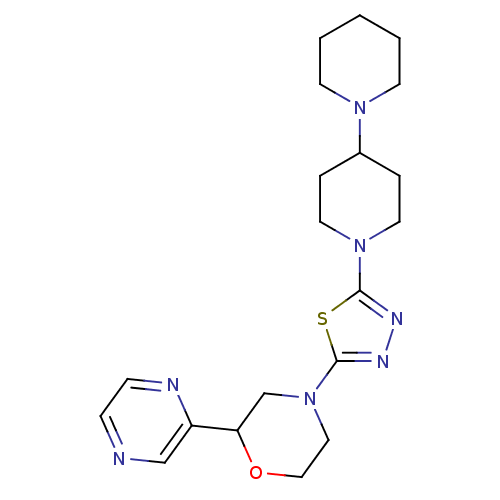 (CHEMBL2048595) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50386359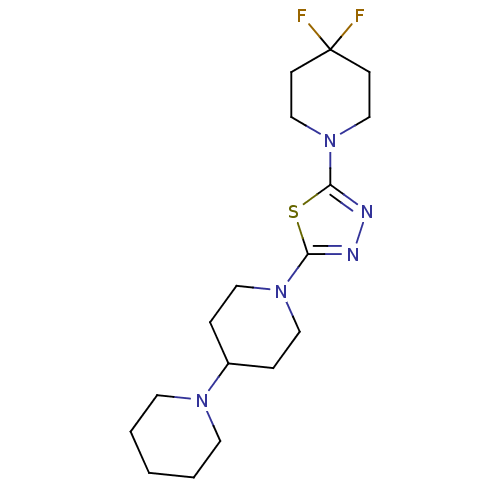 (CHEMBL2048581) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from mouse recombinant histamine H3 receptor after 30 mins by scintillation counting | ACS Med Chem Lett 3: 198-202 (2012) Article DOI: 10.1021/ml200250t BindingDB Entry DOI: 10.7270/Q2736S0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 84 total ) | Next | Last >> |