 Found 12 hits of ki data for polymerid = 50001405
Found 12 hits of ki data for polymerid = 50001405 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50533413
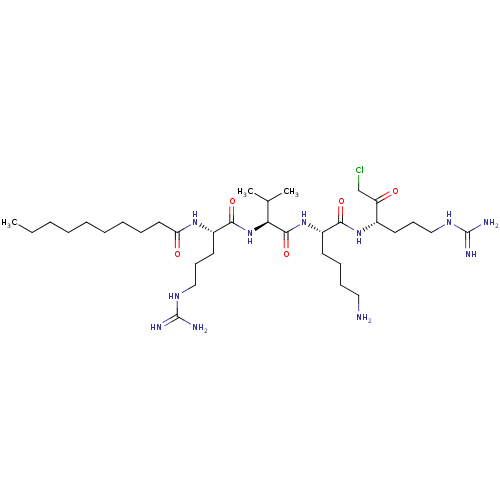
(CHEMBL3126388)Show SMILES CCCCCCCCCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)CCl |r| Show InChI InChI=1S/C34H66ClN11O5/c1-4-5-6-7-8-9-10-18-28(48)43-25(17-14-21-42-34(39)40)31(50)46-29(23(2)3)32(51)45-26(15-11-12-19-36)30(49)44-24(27(47)22-35)16-13-20-41-33(37)38/h23-26,29H,4-22,36H2,1-3H3,(H,43,48)(H,44,49)(H,45,51)(H,46,50)(H4,37,38,41)(H4,39,40,42)/t24-,25-,26-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal truncated human SPC6 expressed in drosophila Schneider 2 cells after 1 hr by spectrofluorometry |
J Med Chem 59: 7719-37 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01516
BindingDB Entry DOI: 10.7270/Q2M048X4 |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50303774
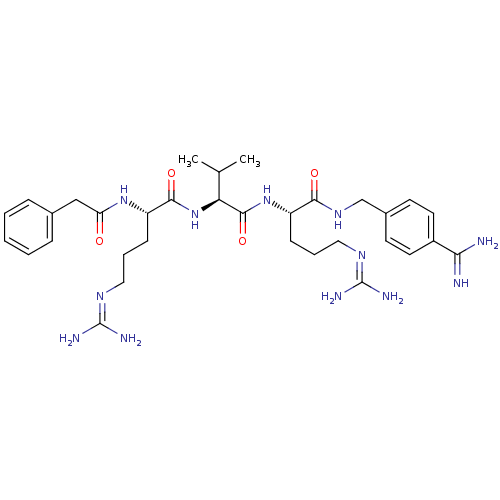
(CHEMBL566340 | phenylacetyl-Arg-Val-Arg-4-amidinob...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C33H50N12O4/c1-20(2)27(45-30(48)25(11-7-17-41-33(38)39)43-26(46)18-21-8-4-3-5-9-21)31(49)44-24(10-6-16-40-32(36)37)29(47)42-19-22-12-14-23(15-13-22)28(34)35/h3-5,8-9,12-15,20,24-25,27H,6-7,10-11,16-19H2,1-2H3,(H3,34,35)(H,42,47)(H,43,46)(H,44,49)(H,45,48)(H4,36,37,40)(H4,38,39,41)/t24-,25-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human PC5/6 expressed in Drosophila schneider 2 cells by fluorescence assay |
J Med Chem 53: 1067-75 (2010)
Article DOI: 10.1021/jm9012455
BindingDB Entry DOI: 10.7270/Q2DN455Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50303775

((S)-2-acetamido-N-((S)-1-((S)-1-(4-carbamimidoylbe...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C27H46N12O4/c1-15(2)21(39-24(42)20(37-16(3)40)7-5-13-35-27(32)33)25(43)38-19(6-4-12-34-26(30)31)23(41)36-14-17-8-10-18(11-9-17)22(28)29/h8-11,15,19-21H,4-7,12-14H2,1-3H3,(H3,28,29)(H,36,41)(H,37,40)(H,38,43)(H,39,42)(H4,30,31,34)(H4,32,33,35)/t19-,20-,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human PC5/6 expressed in Drosophila schneider 2 cells by fluorescence assay |
J Med Chem 53: 1067-75 (2010)
Article DOI: 10.1021/jm9012455
BindingDB Entry DOI: 10.7270/Q2DN455Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50303776
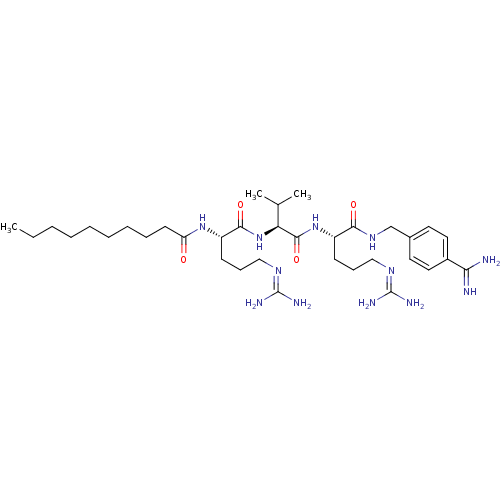
(CHEMBL568525 | N-((6S,9S,12S)-1,17-diamino-6-(4-ca...)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C35H62N12O4/c1-4-5-6-7-8-9-10-15-28(48)45-27(14-12-21-43-35(40)41)32(50)47-29(23(2)3)33(51)46-26(13-11-20-42-34(38)39)31(49)44-22-24-16-18-25(19-17-24)30(36)37/h16-19,23,26-27,29H,4-15,20-22H2,1-3H3,(H3,36,37)(H,44,49)(H,45,48)(H,46,51)(H,47,50)(H4,38,39,42)(H4,40,41,43)/t26-,27-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human PC5/6 expressed in Drosophila schneider 2 cells by fluorescence assay |
J Med Chem 53: 1067-75 (2010)
Article DOI: 10.1021/jm9012455
BindingDB Entry DOI: 10.7270/Q2DN455Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50303777
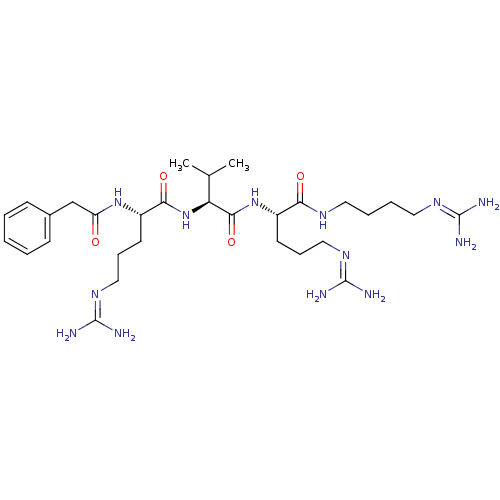
((S)-5-guanidino-N-((S)-1-((S)-5-guanidino-1-(3-gua...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7] |r| Show InChI InChI=1S/C30H53N13O4/c1-19(2)24(27(47)42-21(12-8-16-39-29(33)34)25(45)37-14-6-7-15-38-28(31)32)43-26(46)22(13-9-17-40-30(35)36)41-23(44)18-20-10-4-3-5-11-20/h3-5,10-11,19,21-22,24H,6-9,12-18H2,1-2H3,(H,37,45)(H,41,44)(H,42,47)(H,43,46)(H4,31,32,38)(H4,33,34,39)(H4,35,36,40)/t21-,22-,24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human PC5/6 expressed in Drosophila schneider 2 cells by fluorescence assay |
J Med Chem 53: 1067-75 (2010)
Article DOI: 10.1021/jm9012455
BindingDB Entry DOI: 10.7270/Q2DN455Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50303778
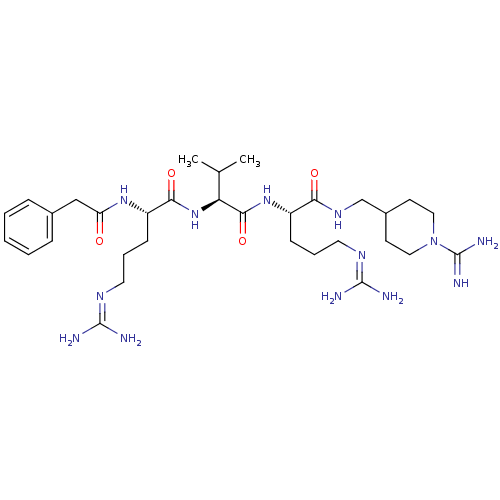
((S)-N-((1-carbamimidoylpiperidin-4-yl)methyl)-5-gu...)Show SMILES [#6]-[#6](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C32H55N13O4/c1-20(2)26(44-28(48)24(11-7-15-40-31(35)36)42-25(46)18-21-8-4-3-5-9-21)29(49)43-23(10-6-14-39-30(33)34)27(47)41-19-22-12-16-45(17-13-22)32(37)38/h3-5,8-9,20,22-24,26H,6-7,10-19H2,1-2H3,(H3,37,38)(H,41,47)(H,42,46)(H,43,49)(H,44,48)(H4,33,34,39)(H4,35,36,40)/t23-,24-,26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human PC5/6 expressed in Drosophila schneider 2 cells by fluorescence assay |
J Med Chem 53: 1067-75 (2010)
Article DOI: 10.1021/jm9012455
BindingDB Entry DOI: 10.7270/Q2DN455Q |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50270060
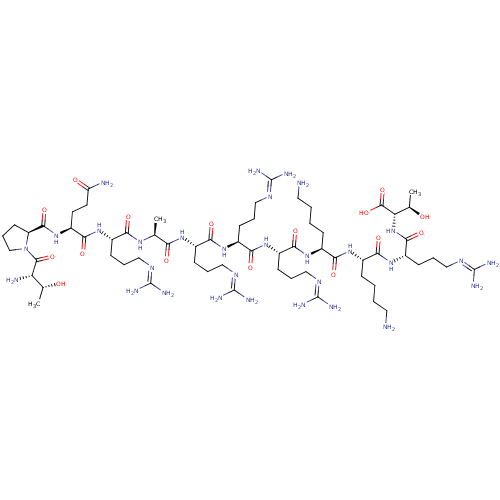
(CHEMBL455792 | TPQRARRRKKRT)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](-[#8])=O |r| Show InChI InChI=1S/C63H120N30O16/c1-32(83-48(98)37(16-8-26-78-59(68)69)87-54(104)42(22-23-44(66)96)91-56(106)43-21-13-31-93(43)57(107)45(67)33(2)94)47(97)84-38(17-9-27-79-60(70)71)51(101)88-40(19-11-29-81-62(74)75)53(103)89-39(18-10-28-80-61(72)73)52(102)86-35(14-4-6-24-64)49(99)85-36(15-5-7-25-65)50(100)90-41(20-12-30-82-63(76)77)55(105)92-46(34(3)95)58(108)109/h32-43,45-46,94-95H,4-31,64-65,67H2,1-3H3,(H2,66,96)(H,83,98)(H,84,97)(H,85,99)(H,86,102)(H,87,104)(H,88,101)(H,89,103)(H,90,100)(H,91,106)(H,92,105)(H,108,109)(H4,68,69,78)(H4,70,71,79)(H4,72,73,80)(H4,74,75,81)(H4,76,77,82)/t32-,33+,34+,35-,36-,37-,38-,39-,40-,41-,42-,43-,45-,46-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PC5/6 assessed as fluorescent Pyr-RTKR-AMC substrate cleavage |
J Biol Chem 282: 20847-53 (2007)
Article DOI: 10.1074/jbc.M703847200
BindingDB Entry DOI: 10.7270/Q2057FPB |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50270054

(CHEMBL502642 | TPRARRRKKRT)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6@@H](-[#6])-[#8])-[#6](-[#7])=O |r| Show InChI InChI=1S/C58H113N29O13/c1-30(78-45(92)35(16-8-24-73-54(63)64)85-52(99)40-21-13-29-87(40)53(100)41(61)31(2)88)44(91)79-36(17-9-25-74-55(65)66)48(95)82-38(19-11-27-76-57(69)70)50(97)83-37(18-10-26-75-56(67)68)49(96)81-33(14-4-6-22-59)46(93)80-34(15-5-7-23-60)47(94)84-39(20-12-28-77-58(71)72)51(98)86-42(32(3)89)43(62)90/h30-42,88-89H,4-29,59-61H2,1-3H3,(H2,62,90)(H,78,92)(H,79,91)(H,80,93)(H,81,96)(H,82,95)(H,83,97)(H,84,94)(H,85,99)(H,86,98)(H4,63,64,73)(H4,65,66,74)(H4,67,68,75)(H4,69,70,76)(H4,71,72,77)/t30-,31+,32+,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PC5/6 assessed as fluorescent Pyr-RTKR-AMC substrate cleavage |
J Biol Chem 282: 20847-53 (2007)
Article DOI: 10.1074/jbc.M703847200
BindingDB Entry DOI: 10.7270/Q2057FPB |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50270067
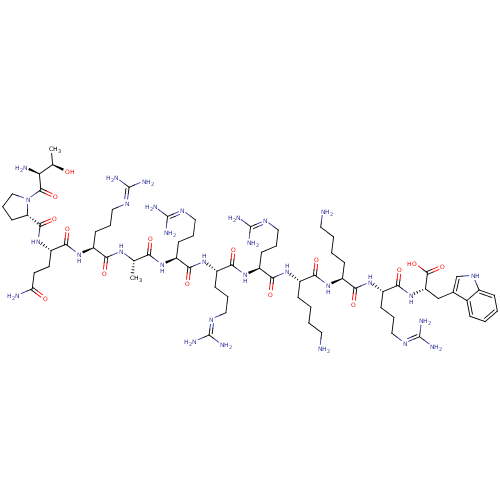
(CHEMBL503520 | TPQRARRRKKRW)Show SMILES C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r,wU:1.1,15.15,35.36,51.51,73.73,91.91,wD:3.3,11.12,24.24,40.40,62.62,82.82,102.102,(-7.97,-22.52,;-8.16,-20.99,;-9.59,-20.4,;-6.94,-20.06,;-7.13,-18.53,;-5.52,-20.65,;-4.3,-19.72,;-5.33,-22.18,;-6.46,-23.42,;-5.63,-24.89,;-3.98,-24.55,;-4.06,-23,;-2.73,-22.24,;-2.73,-20.7,;-1.4,-23,;-.07,-22.24,;-.07,-20.7,;1.27,-19.93,;1.27,-18.39,;-.06,-17.62,;2.6,-17.62,;1.26,-23.01,;1.26,-24.54,;2.59,-22.24,;3.93,-23,;3.93,-24.55,;5.26,-25.31,;5.26,-26.86,;6.59,-27.62,;6.59,-29.17,;5.25,-29.94,;7.92,-29.94,;5.26,-22.24,;5.26,-20.7,;6.59,-23.01,;7.93,-22.24,;7.93,-20.71,;9.26,-23.01,;9.26,-24.55,;10.59,-22.25,;11.93,-23.01,;11.93,-24.56,;13.26,-25.32,;13.26,-26.87,;14.59,-27.63,;14.59,-29.18,;13.25,-29.95,;15.92,-29.94,;13.26,-22.24,;13.26,-20.71,;14.59,-23.02,;15.92,-22.25,;15.92,-20.72,;17.26,-19.95,;17.26,-18.41,;18.59,-17.64,;18.59,-16.11,;17.27,-15.34,;19.93,-15.34,;17.26,-23.02,;17.26,-24.57,;18.59,-22.26,;19.92,-23.03,;19.92,-24.57,;21.25,-25.34,;21.25,-26.88,;22.58,-27.65,;22.58,-29.19,;21.25,-29.96,;23.91,-29.96,;21.25,-22.26,;21.25,-20.73,;22.59,-23.03,;23.92,-22.27,;23.92,-20.73,;25.26,-19.95,;25.26,-18.42,;26.59,-17.65,;26.59,-16.12,;25.26,-23.03,;25.26,-24.57,;26.58,-22.26,;27.92,-23.04,;27.92,-24.58,;29.25,-25.35,;29.25,-26.89,;30.57,-27.66,;30.57,-29.19,;29.25,-22.27,;29.25,-20.74,;30.58,-23.04,;31.91,-22.28,;31.91,-20.73,;33.25,-19.96,;33.25,-18.43,;34.58,-17.66,;34.58,-16.12,;33.26,-15.36,;35.92,-15.35,;33.25,-23.04,;33.25,-24.58,;34.57,-22.27,;35.91,-23.04,;35.91,-24.59,;37.24,-25.36,;38.65,-24.73,;39.68,-25.88,;38.9,-27.21,;39.38,-28.67,;38.34,-29.82,;36.84,-29.49,;36.37,-28.04,;37.4,-26.89,;37.24,-22.28,;38.57,-23.05,;37.24,-20.74,)| Show InChI InChI=1S/C70H123N31O15/c1-37(91-55(105)44(19-9-29-85-66(75)76)95-62(112)49(25-26-52(73)103)99-63(113)51-24-14-34-101(51)64(114)53(74)38(2)102)54(104)92-45(20-10-30-86-67(77)78)58(108)96-47(22-12-32-88-69(81)82)60(110)97-46(21-11-31-87-68(79)80)59(109)94-42(17-5-7-27-71)56(106)93-43(18-6-8-28-72)57(107)98-48(23-13-33-89-70(83)84)61(111)100-50(65(115)116)35-39-36-90-41-16-4-3-15-40(39)41/h3-4,15-16,36-38,42-51,53,90,102H,5-14,17-35,71-72,74H2,1-2H3,(H2,73,103)(H,91,105)(H,92,104)(H,93,106)(H,94,109)(H,95,112)(H,96,108)(H,97,110)(H,98,107)(H,99,113)(H,100,111)(H,115,116)(H4,75,76,85)(H4,77,78,86)(H4,79,80,87)(H4,81,82,88)(H4,83,84,89)/t37-,38+,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,53-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 433 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PC5/6 assessed as fluorescent Pyr-RTKR-AMC substrate cleavage |
J Biol Chem 282: 20847-53 (2007)
Article DOI: 10.1074/jbc.M703847200
BindingDB Entry DOI: 10.7270/Q2057FPB |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50269901
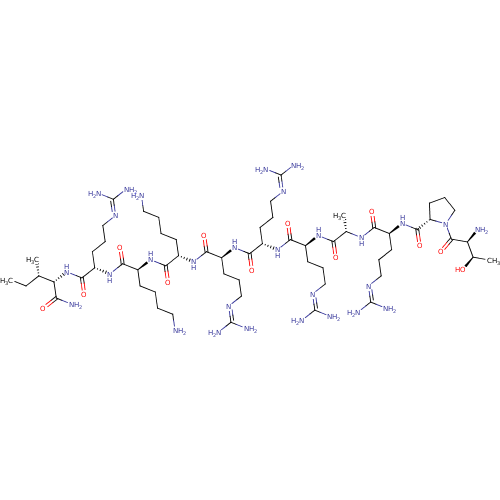
(CHEMBL525748 | TPRARRRKKRI)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6@@H](-[#6])-[#8])-[#6](-[#7])=O |r| Show InChI InChI=1S/C60H117N29O12/c1-5-32(2)44(45(64)91)88-53(99)41(22-14-30-79-60(73)74)86-49(95)36(17-7-9-25-62)82-48(94)35(16-6-8-24-61)83-51(97)39(20-12-28-77-58(69)70)85-52(98)40(21-13-29-78-59(71)72)84-50(96)38(19-11-27-76-57(67)68)81-46(92)33(3)80-47(93)37(18-10-26-75-56(65)66)87-54(100)42-23-15-31-89(42)55(101)43(63)34(4)90/h32-44,90H,5-31,61-63H2,1-4H3,(H2,64,91)(H,80,93)(H,81,92)(H,82,94)(H,83,97)(H,84,96)(H,85,98)(H,86,95)(H,87,100)(H,88,99)(H4,65,66,75)(H4,67,68,76)(H4,69,70,77)(H4,71,72,78)(H4,73,74,79)/t32-,33-,34+,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 492 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PC5/6 assessed as fluorescent Pyr-RTKR-AMC substrate cleavage |
J Biol Chem 282: 20847-53 (2007)
Article DOI: 10.1074/jbc.M703847200
BindingDB Entry DOI: 10.7270/Q2057FPB |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50270069

(CHEMBL499438 | TPQRARRRKKRY)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C68H122N30O16/c1-36(88-53(103)42(15-7-29-83-64(73)74)92-60(110)47(25-26-50(71)101)96-61(111)49-20-12-34-98(49)62(112)51(72)37(2)99)52(102)89-43(16-8-30-84-65(75)76)56(106)93-45(18-10-32-86-67(79)80)58(108)94-44(17-9-31-85-66(77)78)57(107)91-40(13-3-5-27-69)54(104)90-41(14-4-6-28-70)55(105)95-46(19-11-33-87-68(81)82)59(109)97-48(63(113)114)35-38-21-23-39(100)24-22-38/h21-24,36-37,40-49,51,99-100H,3-20,25-35,69-70,72H2,1-2H3,(H2,71,101)(H,88,103)(H,89,102)(H,90,104)(H,91,107)(H,92,110)(H,93,106)(H,94,108)(H,95,105)(H,96,111)(H,97,109)(H,113,114)(H4,73,74,83)(H4,75,76,84)(H4,77,78,85)(H4,79,80,86)(H4,81,82,87)/t36-,37+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 649 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PC5/6 assessed as fluorescent Pyr-RTKR-AMC substrate cleavage |
J Biol Chem 282: 20847-53 (2007)
Article DOI: 10.1074/jbc.M703847200
BindingDB Entry DOI: 10.7270/Q2057FPB |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50270068
(CHEMBL500184 | TPQRARRRKKRF)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C68H122N30O15/c1-37(88-53(102)42(20-10-30-83-64(73)74)92-60(109)47(26-27-50(71)100)96-61(110)49-25-15-35-98(49)62(111)51(72)38(2)99)52(101)89-43(21-11-31-84-65(75)76)56(105)93-45(23-13-33-86-67(79)80)58(107)94-44(22-12-32-85-66(77)78)57(106)91-40(18-6-8-28-69)54(103)90-41(19-7-9-29-70)55(104)95-46(24-14-34-87-68(81)82)59(108)97-48(63(112)113)36-39-16-4-3-5-17-39/h3-5,16-17,37-38,40-49,51,99H,6-15,18-36,69-70,72H2,1-2H3,(H2,71,100)(H,88,102)(H,89,101)(H,90,103)(H,91,106)(H,92,109)(H,93,105)(H,94,107)(H,95,104)(H,96,110)(H,97,108)(H,112,113)(H4,73,74,83)(H4,75,76,84)(H4,77,78,85)(H4,79,80,86)(H4,81,82,87)/t37-,38+,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 806 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PC5/6 assessed as fluorescent Pyr-RTKR-AMC substrate cleavage |
J Biol Chem 282: 20847-53 (2007)
Article DOI: 10.1074/jbc.M703847200
BindingDB Entry DOI: 10.7270/Q2057FPB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data











