 Found 74 hits of ic50 data for polymerid = 50001579
Found 74 hits of ic50 data for polymerid = 50001579 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50299218

(8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...)Show SMILES COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)| Show InChI InChI=1S/C22H19N5O4/c1-12-8-9-13(28)10-14(12)16-11-26-18-19(25(2)22(30)24-20(18)29)23-21(26)27(16)15-6-4-5-7-17(15)31-3/h4-11,28H,1-3H3,(H,24,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 by [gamma33-P]ATP based assay |
J Med Chem 52: 6433-46 (2009)
Article DOI: 10.1021/jm9009444
BindingDB Entry DOI: 10.7270/Q2BZ663G |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50536679
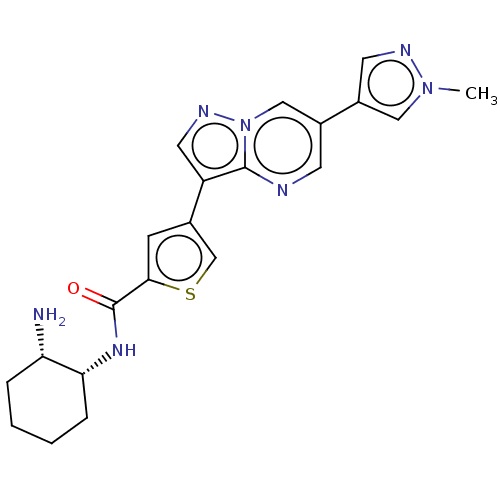
(CHEMBL4568087)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)N[C@@H]1CCCC[C@@H]1N |r| Show InChI InChI=1S/C21H23N7OS/c1-27-10-15(8-24-27)14-7-23-20-16(9-25-28(20)11-14)13-6-19(30-12-13)21(29)26-18-5-3-2-4-17(18)22/h6-12,17-18H,2-5,22H2,1H3,(H,26,29)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged cytoplasmic EPHB2 expressed in baculovirus expression system by Z'-LYTE assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of EPHB2 |
Leukemia 23: 477-85 (2009)
Article DOI: 10.1038/leu.2008.334
BindingDB Entry DOI: 10.7270/Q22Z15R6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM4552

(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of EPHB2 |
Leukemia 23: 477-85 (2009)
Article DOI: 10.1038/leu.2008.334
BindingDB Entry DOI: 10.7270/Q22Z15R6 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM378887
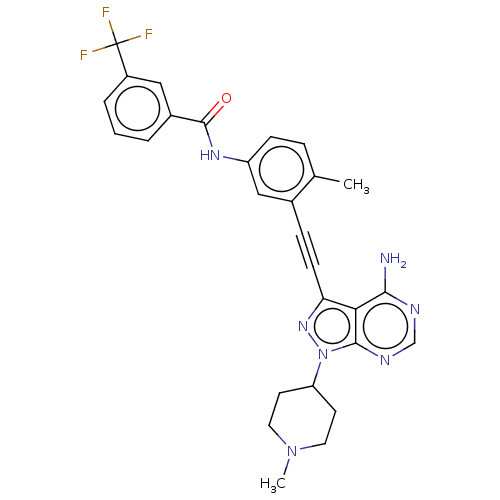
(US10266537, Compound 31)Show SMILES CN1CCC(CC1)n1nc(C#Cc2cc(NC(=O)c3cccc(c3)C(F)(F)F)ccc2C)c2c(N)ncnc12 Show InChI InChI=1S/C28H26F3N7O/c1-17-6-8-21(35-27(39)19-4-3-5-20(14-19)28(29,30)31)15-18(17)7-9-23-24-25(32)33-16-34-26(24)38(36-23)22-10-12-37(2)13-11-22/h3-6,8,14-16,22H,10-13H2,1-2H3,(H,35,39)(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50536675
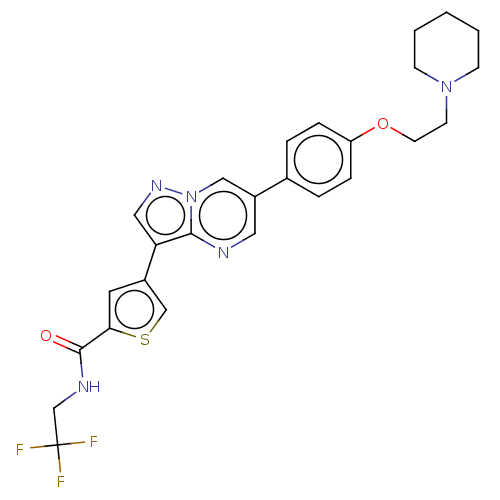
(CHEMBL4569508)Show SMILES FC(F)(F)CNC(=O)c1cc(cs1)-c1cnn2cc(cnc12)-c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C26H26F3N5O2S/c27-26(28,29)17-31-25(35)23-12-19(16-37-23)22-14-32-34-15-20(13-30-24(22)34)18-4-6-21(7-5-18)36-11-10-33-8-2-1-3-9-33/h4-7,12-16H,1-3,8-11,17H2,(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged cytoplasmic EPHB2 expressed in baculovirus expression system by Z'-LYTE assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50331095
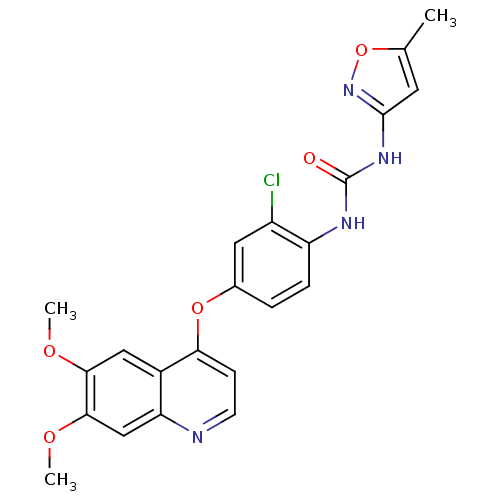
(CHEMBL1289494 | Tivozanib | US10464902, Tivozanib)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)Nc4cc(C)on4)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C22H19ClN4O5/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18/h4-11H,1-3H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant EphB2 (unknown origin) by cell-free assay |
Bioorg Med Chem Lett 25: 2425-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.088
BindingDB Entry DOI: 10.7270/Q2KW5HRT |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human EphB2 |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50331095

(CHEMBL1289494 | Tivozanib | US10464902, Tivozanib)Show SMILES COc1cc2nccc(Oc3ccc(NC(=O)Nc4cc(C)on4)c(Cl)c3)c2cc1OC Show InChI InChI=1S/C22H19ClN4O5/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18/h4-11H,1-3H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant EphB2 (unknown origin) by cell-free assay |
Bioorg Med Chem Lett 25: 2425-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.088
BindingDB Entry DOI: 10.7270/Q2KW5HRT |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc.
| Assay Description
The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... |
Bioorg Med Chem Lett 17: 3562-9 (2007)
BindingDB Entry DOI: 10.7270/Q21C2065 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50536678
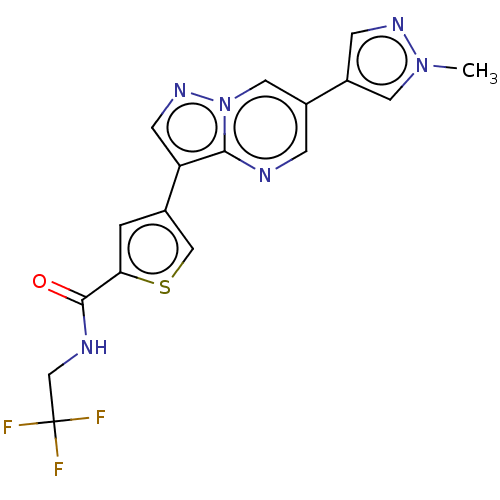
(CHEMBL4550702)Show SMILES Cn1cc(cn1)-c1cnc2c(cnn2c1)-c1csc(c1)C(=O)NCC(F)(F)F Show InChI InChI=1S/C17H13F3N6OS/c1-25-6-12(4-23-25)11-3-21-15-13(5-24-26(15)7-11)10-2-14(28-8-10)16(27)22-9-17(18,19)20/h2-8H,9H2,1H3,(H,22,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged cytoplasmic EPHB2 expressed in baculovirus expression system by Z'-LYTE assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42.4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human EPHB2 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50538442

(CHEMBL4638981)Show SMILES Cc1ccc(cc1C#Cc1cnc2cccnn12)C(=O)Nc1ccc(CN2Cc3cn[nH]c3C2)c(Cl)c1 Show InChI InChI=1S/C28H22ClN7O/c1-18-4-5-20(11-19(18)7-9-24-14-30-27-3-2-10-32-36(24)27)28(37)33-23-8-6-21(25(29)12-23)15-35-16-22-13-31-34-26(22)17-35/h2-6,8,10-14H,15-17H2,1H3,(H,31,34)(H,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of EPHB2 (unknown origin) assessed as residual activity incubated for 5 mins in presence of [gamma-33ATP] by scintillation counting based ... |
ACS Med Chem Lett 11: 491-496 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00612
BindingDB Entry DOI: 10.7270/Q2377D68 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50328755

(2-(3-(3-tert-butyl-5-(3-(2,3-dichlorophenyl)ureido...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2cccc(Cl)c2Cl)n(n1)-c1cccc(CC(N)=O)c1 Show InChI InChI=1S/C22H23Cl2N5O2/c1-22(2,3)17-12-19(27-21(31)26-16-9-5-8-15(23)20(16)24)29(28-17)14-7-4-6-13(10-14)11-18(25)30/h4-10,12H,11H2,1-3H3,(H2,25,30)(H2,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Deciphera Pharmaceuticals LLC
Curated by ChEMBL
| Assay Description
Inhibition of EPHB2 |
Bioorg Med Chem Lett 20: 5793-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.134
BindingDB Entry DOI: 10.7270/Q2XD11WK |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50538438

(CHEMBL4640297)Show SMILES Cc1ccc(cc1C#Cc1cnc2cccnn12)C(=O)Nc1ccc(CN2CCC3C=NNC3C2)c(Cl)c1 |c:33| Show InChI InChI=1S/C29H26ClN7O/c1-19-4-5-21(13-20(19)7-9-25-16-31-28-3-2-11-33-37(25)28)29(38)34-24-8-6-23(26(30)14-24)17-36-12-10-22-15-32-35-27(22)18-36/h2-6,8,11,13-16,22,27,35H,10,12,17-18H2,1H3,(H,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of EPHB2 (unknown origin) assessed as residual activity incubated for 5 mins in presence of [gamma-33ATP] by scintillation counting based ... |
ACS Med Chem Lett 11: 491-496 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00612
BindingDB Entry DOI: 10.7270/Q2377D68 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB2 using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of EPHB2 |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University
Curated by ChEMBL
| Assay Description
Inhibition of EPHB2 (unknown origin) incubated for 1 hr by spectrophotometric analysis |
Bioorg Med Chem 24: 3483-93 (2016)
Article DOI: 10.1016/j.bmc.2016.05.057
BindingDB Entry DOI: 10.7270/Q29G5PQT |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50498455

(CHEMBL3596879)Show SMILES Cn1cc(cn1)-c1cncc(Oc2cccc(NC(=O)Nc3cc(on3)C(C)(C)C)c2)n1 Show InChI InChI=1S/C22H23N7O3/c1-22(2,3)18-9-19(28-32-18)27-21(30)25-15-6-5-7-16(8-15)31-20-12-23-11-17(26-20)14-10-24-29(4)13-14/h5-13H,1-4H3,(H2,25,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 (unknown origin) using ATP and 5FAM tagged TrkA peptide substrate incubated for 60 mins by microfluidics assay |
Medchemcomm 5: 1507-1514 (2014)
Article DOI: 10.1039/c4md00251b
BindingDB Entry DOI: 10.7270/Q2WW7MQ5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50462709
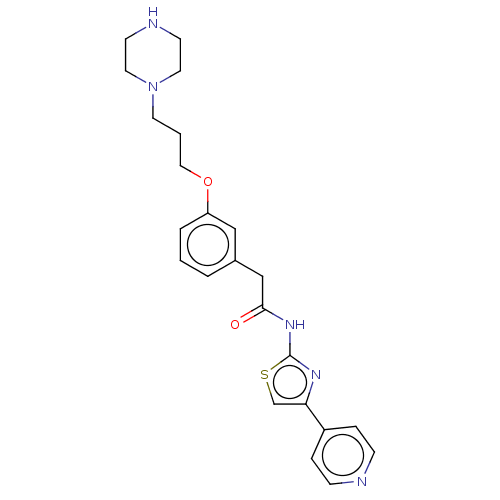
(CHEMBL4245507)Show SMILES O=C(Cc1cccc(OCCCN2CCNCC2)c1)Nc1nc(cs1)-c1ccncc1 Show InChI InChI=1S/C23H27N5O2S/c29-22(27-23-26-21(17-31-23)19-5-7-24-8-6-19)16-18-3-1-4-20(15-18)30-14-2-11-28-12-9-25-10-13-28/h1,3-8,15,17,25H,2,9-14,16H2,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of EPH-B2 (unknown origin) |
Bioorg Med Chem Lett 28: 2616-2621 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.043
BindingDB Entry DOI: 10.7270/Q2XP77MH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 62: 5298-5311 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00058
BindingDB Entry DOI: 10.7270/Q24170ZD |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50563891
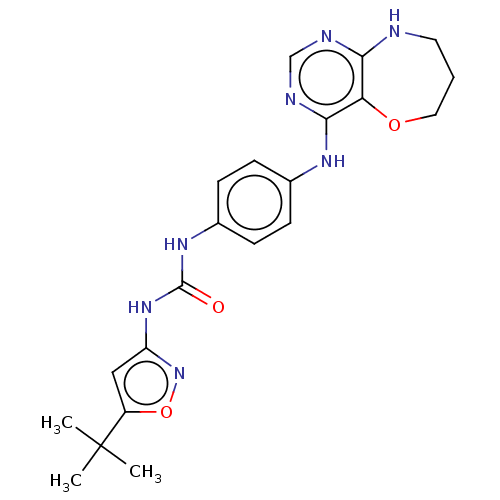
(CHEMBL4793380)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(Nc3ncnc4NCCCOc34)cc2)no1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human EphB2 (560 to end residues) using poly(Glu, Tyr) 4:1 as substrate incubated for 40 mins in presence of [gamma-33ATP] ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.09.018
BindingDB Entry DOI: 10.7270/Q270854P |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM4622
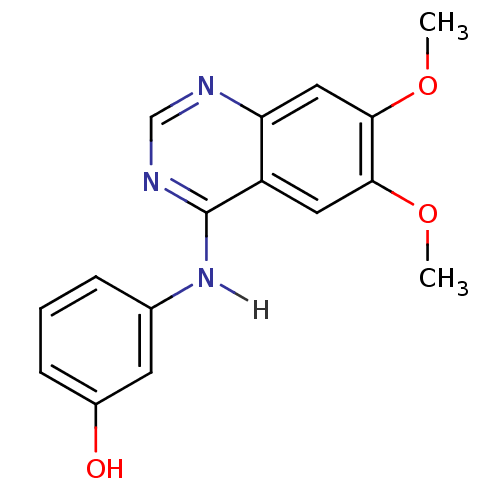
(3-[(6,7-dimethoxyquinazolin-4-yl)amino]phenol | An...)Show InChI InChI=1S/C16H15N3O3/c1-21-14-7-12-13(8-15(14)22-2)17-9-18-16(12)19-10-4-3-5-11(20)6-10/h3-9,20H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Protana Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPH receptor B2 using ELISA |
J Med Chem 48: 3221-30 (2005)
Article DOI: 10.1021/jm0492204
BindingDB Entry DOI: 10.7270/Q2DF6QRN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50592745
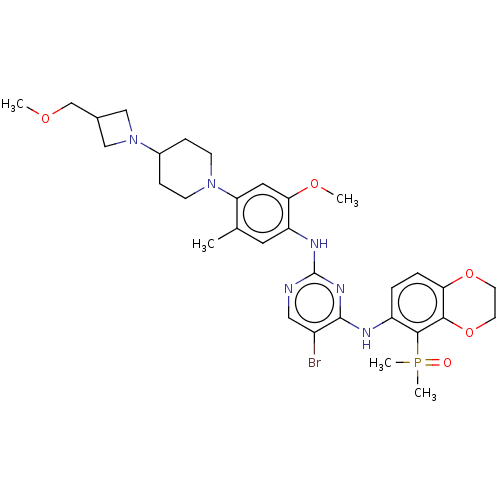
(CHEMBL5188373)Show SMILES COCC1CN(C1)C1CCN(CC1)c1cc(OC)c(Nc2ncc(Br)c(Nc3ccc4OCCOc4c3P(C)(C)=O)n2)cc1C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116907
BindingDB Entry DOI: 10.7270/Q2J96BC1 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50498458

(CHEMBL3596868)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2cccc(Oc3cncc(n3)-c3ccsc3)c2)no1 Show InChI InChI=1S/C22H21N5O3S/c1-22(2,3)18-10-19(27-30-18)26-21(28)24-15-5-4-6-16(9-15)29-20-12-23-11-17(25-20)14-7-8-31-13-14/h4-13H,1-3H3,(H2,24,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 (unknown origin) using ATP and 5FAM tagged TrkA peptide substrate incubated for 60 mins by microfluidics assay |
Medchemcomm 5: 1507-1514 (2014)
Article DOI: 10.1039/c4md00251b
BindingDB Entry DOI: 10.7270/Q2WW7MQ5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50498449
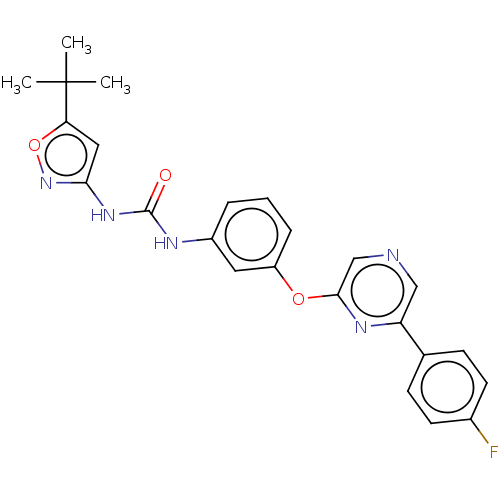
(CHEMBL3596889)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2cccc(Oc3cncc(n3)-c3ccc(F)cc3)c2)no1 Show InChI InChI=1S/C24H22FN5O3/c1-24(2,3)20-12-21(30-33-20)29-23(31)27-17-5-4-6-18(11-17)32-22-14-26-13-19(28-22)15-7-9-16(25)10-8-15/h4-14H,1-3H3,(H2,27,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 (unknown origin) using ATP and 5FAM tagged TrkA peptide substrate incubated for 60 mins by microfluidics assay |
Medchemcomm 5: 1507-1514 (2014)
Article DOI: 10.1039/c4md00251b
BindingDB Entry DOI: 10.7270/Q2WW7MQ5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50498452
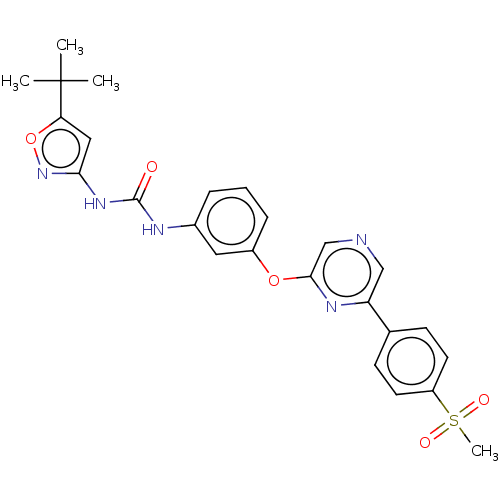
(CHEMBL3596873)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2cccc(Oc3cncc(n3)-c3ccc(cc3)S(C)(=O)=O)c2)no1 Show InChI InChI=1S/C25H25N5O5S/c1-25(2,3)21-13-22(30-35-21)29-24(31)27-17-6-5-7-18(12-17)34-23-15-26-14-20(28-23)16-8-10-19(11-9-16)36(4,32)33/h5-15H,1-4H3,(H2,27,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 (unknown origin) using ATP and 5FAM tagged TrkA peptide substrate incubated for 60 mins by microfluidics assay |
Medchemcomm 5: 1507-1514 (2014)
Article DOI: 10.1039/c4md00251b
BindingDB Entry DOI: 10.7270/Q2WW7MQ5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50498453

(CHEMBL3596862)Show SMILES COc1ccc(cc1)-c1cncc(Oc2cccc(NC(=O)Nc3cc(on3)C(C)(C)C)c2)n1 Show InChI InChI=1S/C25H25N5O4/c1-25(2,3)21-13-22(30-34-21)29-24(31)27-17-6-5-7-19(12-17)33-23-15-26-14-20(28-23)16-8-10-18(32-4)11-9-16/h5-15H,1-4H3,(H2,27,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 (unknown origin) using ATP and 5FAM tagged TrkA peptide substrate incubated for 60 mins by microfluidics assay |
Medchemcomm 5: 1507-1514 (2014)
Article DOI: 10.1039/c4md00251b
BindingDB Entry DOI: 10.7270/Q2WW7MQ5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50208911
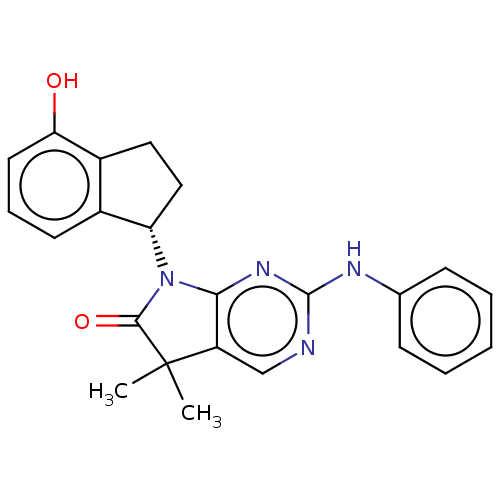
(CHEMBL3884319)Show SMILES CC1(C)C(=O)N([C@H]2CCc3c2cccc3O)c2nc(Nc3ccccc3)ncc12 |r| Show InChI InChI=1S/C23H22N4O2/c1-23(2)17-13-24-22(25-14-7-4-3-5-8-14)26-20(17)27(21(23)29)18-12-11-16-15(18)9-6-10-19(16)28/h3-10,13,18,28H,11-12H2,1-2H3,(H,24,25,26)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged EPHB2 cytoplasmic domain (616 to 884 residues) expressed in baculovirus expression system |
Bioorg Med Chem Lett 27: 114-120 (2017)
Article DOI: 10.1016/j.bmcl.2016.08.068
BindingDB Entry DOI: 10.7270/Q2N018JC |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50498457

(CHEMBL3596893)Show SMILES Cn1cc(cn1)-c1cncc(n1)-c1ccc(NC(=O)Nc2cc(nn2C)C(C)(C)C)cc1 Show InChI InChI=1S/C23H26N8O/c1-23(2,3)20-10-21(31(5)29-20)28-22(32)26-17-8-6-15(7-9-17)18-12-24-13-19(27-18)16-11-25-30(4)14-16/h6-14H,1-5H3,(H2,26,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 (unknown origin) using ATP and 5FAM tagged TrkA peptide substrate incubated for 60 mins by microfluidics assay |
Medchemcomm 5: 1507-1514 (2014)
Article DOI: 10.1039/c4md00251b
BindingDB Entry DOI: 10.7270/Q2WW7MQ5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50498459

(CHEMBL3596892)Show SMILES Cn1cc(cn1)-c1cncc(n1)-c1ccc(NC(=O)Nc2cc(no2)C(C)(C)C)cc1 Show InChI InChI=1S/C22H23N7O2/c1-22(2,3)19-9-20(31-28-19)27-21(30)25-16-7-5-14(6-8-16)17-11-23-12-18(26-17)15-10-24-29(4)13-15/h5-13H,1-4H3,(H2,25,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 (unknown origin) using ATP and 5FAM tagged TrkA peptide substrate incubated for 60 mins by microfluidics assay |
Medchemcomm 5: 1507-1514 (2014)
Article DOI: 10.1039/c4md00251b
BindingDB Entry DOI: 10.7270/Q2WW7MQ5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50428059
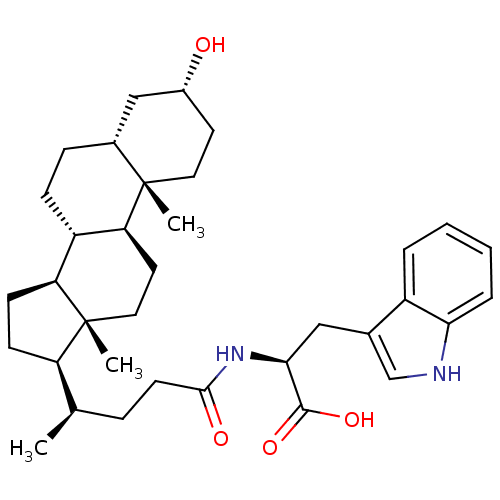
(CHEMBL2322989)Show SMILES C[C@H](CCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C35H50N2O4/c1-21(8-13-32(39)37-31(33(40)41)18-22-20-36-30-7-5-4-6-25(22)30)27-11-12-28-26-10-9-23-19-24(38)14-16-34(23,2)29(26)15-17-35(27,28)3/h4-7,20-21,23-24,26-29,31,36,38H,8-19H2,1-3H3,(H,37,39)(H,40,41)/t21-,23-,24-,26+,27-,28+,29+,31+,34+,35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma
Curated by ChEMBL
| Assay Description
Displacement of ephrin-B1-Fc from EphB2 receptor Fc ectodomain (unknown origin) after 1 hr by ELISA |
J Med Chem 56: 2936-47 (2013)
Article DOI: 10.1021/jm301890k
BindingDB Entry DOI: 10.7270/Q2JW8G7H |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50536681
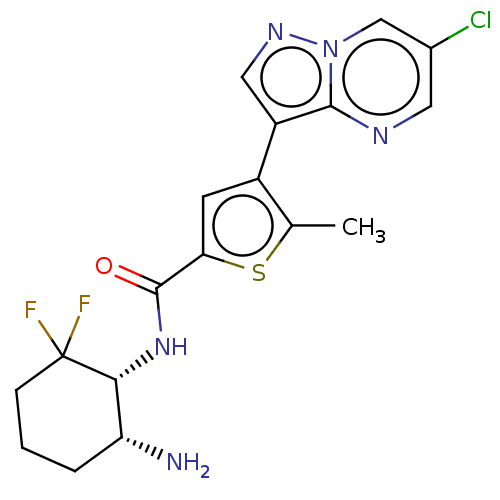
(CHEMBL4552628)Show SMILES Cc1sc(cc1-c1cnn2cc(Cl)cnc12)C(=O)N[C@@H]1[C@H](N)CCCC1(F)F |r| Show InChI InChI=1S/C18H18ClF2N5OS/c1-9-11(12-7-24-26-8-10(19)6-23-16(12)26)5-14(28-9)17(27)25-15-13(22)3-2-4-18(15,20)21/h5-8,13,15H,2-4,22H2,1H3,(H,25,27)/t13-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged cytoplasmic EPHB2 expressed in baculovirus expression system by Z'-LYTE assay |
Bioorg Med Chem Lett 26: 4362-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.003
BindingDB Entry DOI: 10.7270/Q2NP27X5 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM39299
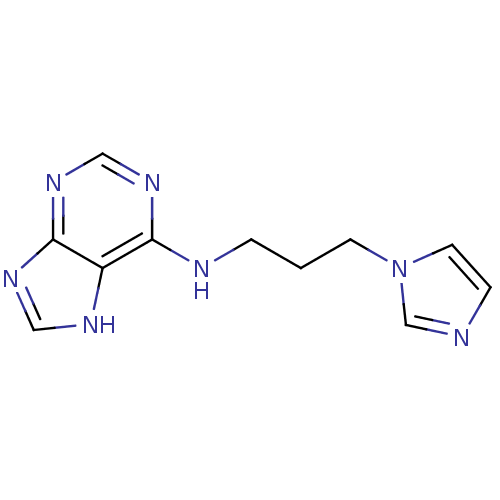
(3-imidazol-1-ylpropyl(7H-purin-6-yl)amine | CHEMBL...)Show InChI InChI=1S/C11H13N7/c1(4-18-5-3-12-8-18)2-13-10-9-11(15-6-14-9)17-7-16-10/h3,5-8H,1-2,4H2,(H2,13,14,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Protana Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPH receptor B2 using ELISA |
J Med Chem 48: 3221-30 (2005)
Article DOI: 10.1021/jm0492204
BindingDB Entry DOI: 10.7270/Q2DF6QRN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50359359

(CHEMBL1929238)Show SMILES CN(C)CCN1CCN(CCC1=O)C(=O)c1cc(sc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C25H33Cl2N5O3S/c1-25(2,3)19-15-16(22(36-19)29-24(35)28-18-8-6-7-17(26)21(18)27)23(34)32-10-9-20(33)31(13-14-32)12-11-30(4)5/h6-8,15H,9-14H2,1-5H3,(H2,28,29,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of EPHB2 |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13336
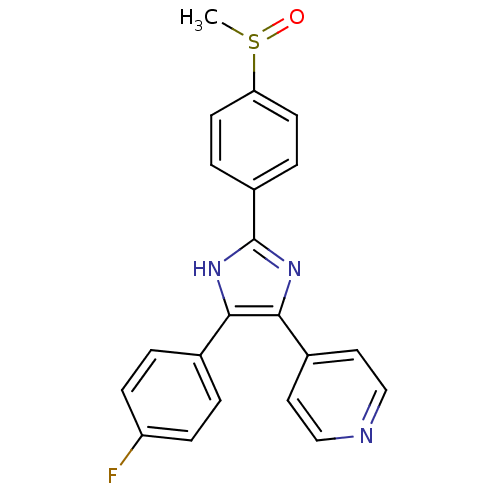
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Protana Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPH receptor B2 using ELISA |
J Med Chem 48: 3221-30 (2005)
Article DOI: 10.1021/jm0492204
BindingDB Entry DOI: 10.7270/Q2DF6QRN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM4621

(Anilinoquinazoline deriv. 4 | CHEMBL150315 | N-(4-...)Show InChI InChI=1S/C16H13ClFN3O2/c1-22-14-6-10-13(7-15(14)23-2)19-8-20-16(10)21-12-4-3-9(17)5-11(12)18/h3-8H,1-2H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Protana Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPH receptor B2 using ELISA |
J Med Chem 48: 3221-30 (2005)
Article DOI: 10.1021/jm0492204
BindingDB Entry DOI: 10.7270/Q2DF6QRN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50263183
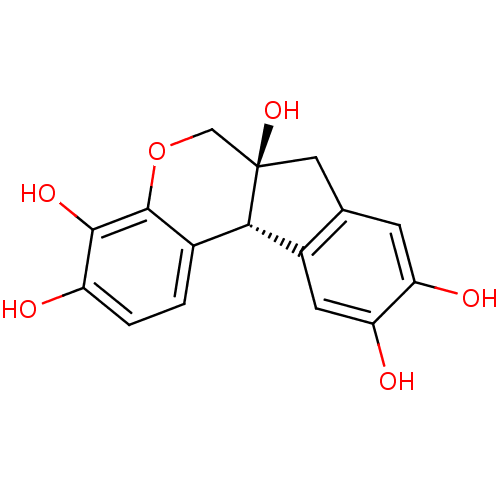
((+)-haematoxylin | (+)-hematoxylin | (6aS,11bR)-7,...)Show SMILES Oc1ccc2[C@H]3c4cc(O)c(O)cc4C[C@@]3(O)COc2c1O |r| Show InChI InChI=1S/C16H14O6/c17-10-2-1-8-13-9-4-12(19)11(18)3-7(9)5-16(13,21)6-22-15(8)14(10)20/h1-4,13,17-21H,5-6H2/t13-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 (unknown origin) by ELISA |
J Med Chem 51: 4419-29 (2008)
Article DOI: 10.1021/jm701501x
BindingDB Entry DOI: 10.7270/Q2N879MQ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50123803
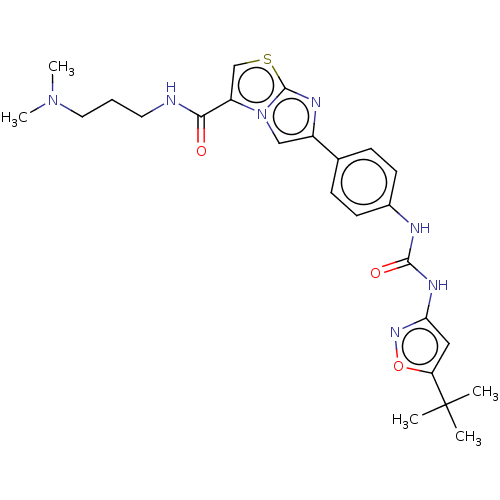
(CHEMBL3623375)Show SMILES CN(C)CCCNC(=O)c1csc2nc(cn12)-c1ccc(NC(=O)Nc2cc(on2)C(C)(C)C)cc1 Show InChI InChI=1S/C25H31N7O3S/c1-25(2,3)20-13-21(30-35-20)29-23(34)27-17-9-7-16(8-10-17)18-14-32-19(15-36-24(32)28-18)22(33)26-11-6-12-31(4)5/h7-10,13-15H,6,11-12H2,1-5H3,(H,26,33)(H2,27,29,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant EphB2 |
Bioorg Med Chem Lett 25: 4534-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.08.068
BindingDB Entry DOI: 10.7270/Q24X59MV |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50135286

(CHEMBL3745885)Show SMILES Cn1c2nc(Nc3ccc4[nH]ccc4c3)ncc2cc(c1=O)S(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C22H15F2N5O3S/c1-29-20-13(9-19(21(29)30)33(31,32)18-5-2-14(23)10-16(18)24)11-26-22(28-20)27-15-3-4-17-12(8-15)6-7-25-17/h2-11,25H,1H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB2 using poly[Glu:Tyr] (4:1) as substrate |
Bioorg Med Chem 24: 521-44 (2016)
Article DOI: 10.1016/j.bmc.2015.11.045
BindingDB Entry DOI: 10.7270/Q24Q7WT8 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50341628
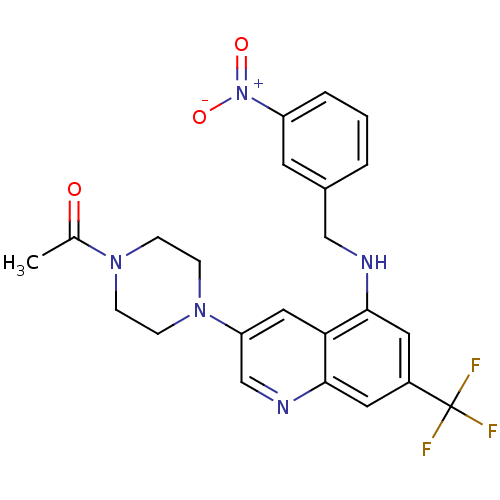
(3-(4-Acetylpiperazin-1-yl)-5-(3-nitrobenzylamino)-...)Show SMILES CC(=O)N1CCN(CC1)c1cnc2cc(cc(NCc3cccc(c3)[N+]([O-])=O)c2c1)C(F)(F)F Show InChI InChI=1S/C23H22F3N5O3/c1-15(32)29-5-7-30(8-6-29)19-12-20-21(10-17(23(24,25)26)11-22(20)28-14-19)27-13-16-3-2-4-18(9-16)31(33)34/h2-4,9-12,14,27H,5-8,13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of EPH-B2 |
J Med Chem 54: 2127-42 (2011)
Article DOI: 10.1021/jm101340q
BindingDB Entry DOI: 10.7270/Q2DR2VTS |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50343726
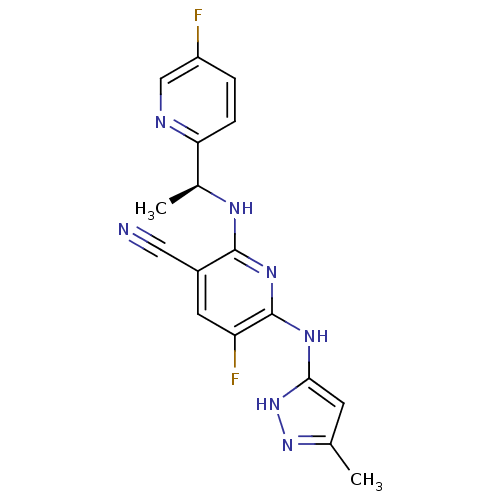
((S)-5-fluoro-2-(1-(5-fluoropyridin-2-yl)ethylamino...)Show SMILES C[C@H](Nc1nc(Nc2cc(C)n[nH]2)c(F)cc1C#N)c1ccc(F)cn1 |r| Show InChI InChI=1S/C17H15F2N7/c1-9-5-15(26-25-9)23-17-13(19)6-11(7-20)16(24-17)22-10(2)14-4-3-12(18)8-21-14/h3-6,8,10H,1-2H3,(H3,22,23,24,25,26)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Inhibition of human EphB2 |
Bioorg Med Chem Lett 21: 2958-61 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.053
BindingDB Entry DOI: 10.7270/Q2PN95ZD |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50345444
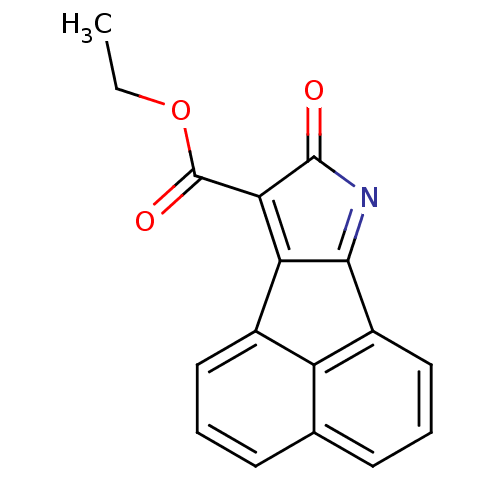
(CHEMBL1784725 | Ethyl 8-oxo-8H-acenaphtho[1,2-b]py...)Show InChI InChI=1S/C17H11NO3/c1-2-21-17(20)14-13-10-7-3-5-9-6-4-8-11(12(9)10)15(13)18-16(14)19/h3-8H,2H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 expressed in Bac-to-Bac Baculovirus expression system using poly(Glu-Tyr)4:1 as substrate after 60 mins by ELISA |
J Med Chem 54: 3732-45 (2011)
Article DOI: 10.1021/jm200258t
BindingDB Entry DOI: 10.7270/Q29W0FTH |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50350069
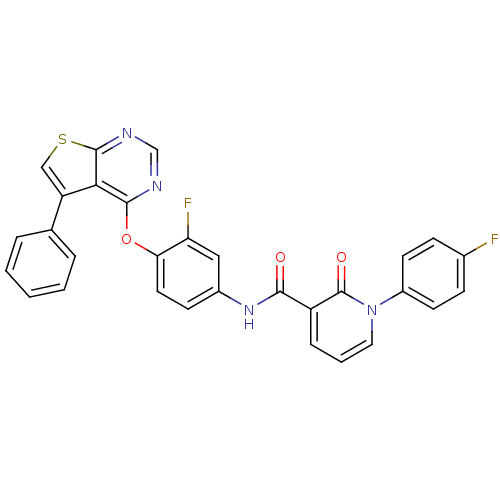
(CHEMBL1813632)Show SMILES Fc1ccc(cc1)-n1cccc(C(=O)Nc2ccc(Oc3ncnc4scc(-c5ccccc5)c34)c(F)c2)c1=O Show InChI InChI=1S/C30H18F2N4O3S/c31-19-8-11-21(12-9-19)36-14-4-7-22(30(36)38)27(37)35-20-10-13-25(24(32)15-20)39-28-26-23(18-5-2-1-3-6-18)16-40-29(26)34-17-33-28/h1-17H,(H,35,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of EPHB2 |
Bioorg Med Chem 19: 3906-18 (2011)
Article DOI: 10.1016/j.bmc.2011.05.038
BindingDB Entry DOI: 10.7270/Q2CF9QGW |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50401152
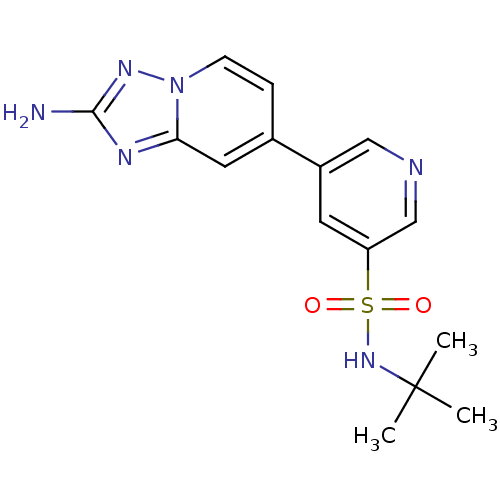
(CHEMBL2205766)Show SMILES CC(C)(C)NS(=O)(=O)c1cncc(c1)-c1ccn2nc(N)nc2c1 Show InChI InChI=1S/C15H18N6O2S/c1-15(2,3)20-24(22,23)12-6-11(8-17-9-12)10-4-5-21-13(7-10)18-14(16)19-21/h4-9,20H,1-3H3,(H2,16,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cellzome Ltd
Curated by ChEMBL
| Assay Description
Inhibition of EPHB2 |
Bioorg Med Chem Lett 22: 4613-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.090
BindingDB Entry DOI: 10.7270/Q2HQ412B |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50321943
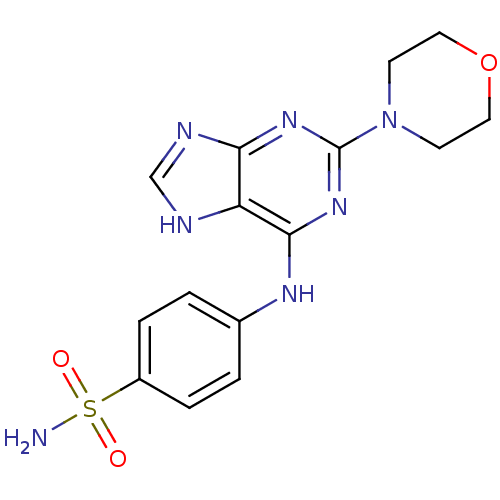
(CHEMBL1172957 | N-(4-Aminosulfonylphenyl)-2-morpho...)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(nc3nc[nH]c23)N2CCOCC2)cc1 Show InChI InChI=1S/C15H17N7O3S/c16-26(23,24)11-3-1-10(2-4-11)19-14-12-13(18-9-17-12)20-15(21-14)22-5-7-25-8-6-22/h1-4,9H,5-8H2,(H2,16,23,24)(H2,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition EphB2 |
Bioorg Med Chem 18: 4615-24 (2011)
Article DOI: 10.1016/j.bmc.2010.05.032
BindingDB Entry DOI: 10.7270/Q26H4JCQ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50041978

(CHEMBL3134157)Show SMILES Cc1cc(CS(=O)(=O)c2ccccc2)cc(OCc2ccc(CN3CCC[C@@H]3CO)cc2)c1 |r| Show InChI InChI=1S/C27H31NO4S/c1-21-14-24(20-33(30,31)27-7-3-2-4-8-27)16-26(15-21)32-19-23-11-9-22(10-12-23)17-28-13-5-6-25(28)18-29/h2-4,7-12,14-16,25,29H,5-6,13,17-20H2,1H3/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00040
BindingDB Entry DOI: 10.7270/Q2959NNX |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50605384

(CHEMBL5175977)Show SMILES Cc1cc(OCc2ccc(CN3CCC[C@@H]3CO)cc2)cc(CS(=O)(=O)c2ccccc2)c1Br |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00040
BindingDB Entry DOI: 10.7270/Q2959NNX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data