 Found 148 hits of kd data for polymerid = 50001579
Found 148 hits of kd data for polymerid = 50001579 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PCBioAssay
| n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2H993KN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50355504
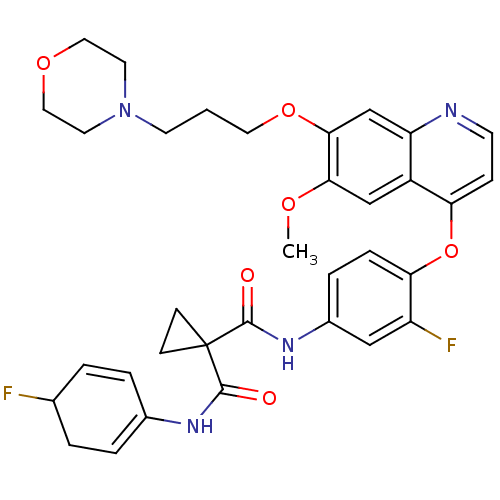
(CHEMBL1908393)Show SMILES COc1cc2c(Oc3ccc(NC(=O)C4(CC4)C(=O)NC4=CCC(F)C=C4)cc3F)ccnc2cc1OCCCN1CCOCC1 |c:26,t:21| Show InChI InChI=1S/C34H36F2N4O6/c1-43-30-20-25-27(21-31(30)45-16-2-13-40-14-17-44-18-15-40)37-12-9-28(25)46-29-8-7-24(19-26(29)36)39-33(42)34(10-11-34)32(41)38-23-5-3-22(35)4-6-23/h3,5-9,12,19-22H,2,4,10-11,13-18H2,1H3,(H,38,41)(H,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM26145
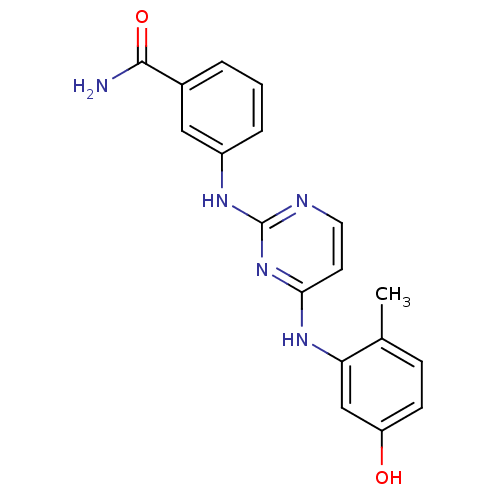
(2,4-dianilino pyrimidine, 2 | 3-({4-[(5-hydroxy-2-...)Show InChI InChI=1S/C18H17N5O2/c1-11-5-6-14(24)10-15(11)22-16-7-8-20-18(23-16)21-13-4-2-3-12(9-13)17(19)25/h2-10,24H,1H3,(H2,19,25)(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human EPHB2 |
J Med Chem 51: 7898-914 (2008)
Article DOI: 10.1021/jm8011036
BindingDB Entry DOI: 10.7270/Q2WS8T4C |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM4552
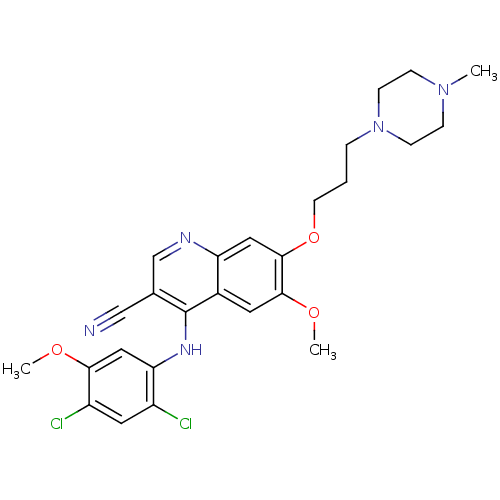
(4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...)Show SMILES COc1cc(Nc2c(cnc3cc(OCCCN4CCN(C)CC4)c(OC)cc23)C#N)c(Cl)cc1Cl Show InChI InChI=1S/C26H29Cl2N5O3/c1-32-6-8-33(9-7-32)5-4-10-36-25-13-21-18(11-24(25)35-3)26(17(15-29)16-30-21)31-22-14-23(34-2)20(28)12-19(22)27/h11-14,16H,4-10H2,1-3H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50468256
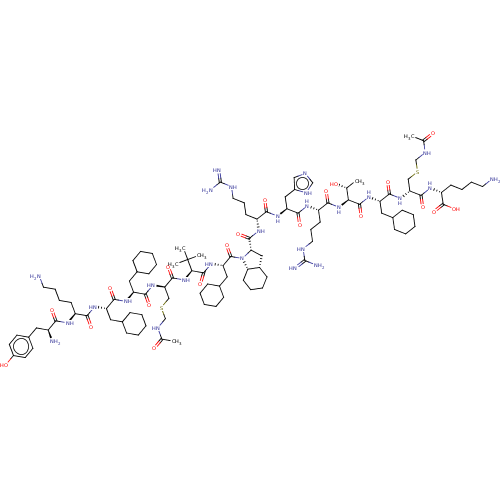
(CHEMBL4276804)Show SMILES [H][C@]12C[C@H](N(C(=O)[C@H](CC3CCCCC3)NC(=O)[C@@H](NC(=O)[C@@H](CSCNC(C)=O)NC(=O)[C@H](CC3CCCCC3)NC(=O)[C@H](CC3CCCCC3)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(C)(C)C)[C@@]1([H])CCCC2)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H](CSCNC(C)=O)C(=O)N[C@H](CCCCN)C(O)=O |r| Show InChI InChI=1S/C106H177N27O20S2/c1-62(134)87(100(149)127-80(52-67-31-15-9-16-32-67)95(144)129-83(57-154-60-118-63(2)135)97(146)123-77(103(152)153)37-22-24-46-108)131-92(141)76(39-26-48-116-105(112)113)121-96(145)81(55-71-56-114-59-117-71)126-91(140)75(38-25-47-115-104(110)111)122-99(148)86-54-70-35-19-20-40-85(70)133(86)102(151)82(53-68-33-17-10-18-34-68)128-101(150)88(106(4,5)6)132-98(147)84(58-155-61-119-64(3)136)130-94(143)79(51-66-29-13-8-14-30-66)125-93(142)78(50-65-27-11-7-12-28-65)124-90(139)74(36-21-23-45-107)120-89(138)73(109)49-69-41-43-72(137)44-42-69/h41-44,56,59,62,65-68,70,73-88,134,137H,7-40,45-55,57-58,60-61,107-109H2,1-6H3,(H,114,117)(H,118,135)(H,119,136)(H,120,138)(H,121,145)(H,122,148)(H,123,146)(H,124,139)(H,125,142)(H,126,140)(H,127,149)(H,128,150)(H,129,144)(H,130,143)(H,131,141)(H,132,147)(H,152,153)(H4,110,111,115)(H4,112,113,116)/t62-,70+,73+,74+,75-,76+,77-,78+,79+,80+,81+,82+,83-,84-,85+,86+,87+,88-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a |
University of Parma
Curated by ChEMBL
| Assay Description
Binding affinity to EphB2 (unknown origin) by SPR assay |
Eur J Med Chem 142: 152-162 (2017)
Article DOI: 10.1016/j.ejmech.2017.07.029
BindingDB Entry DOI: 10.7270/Q29026GX |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM6568
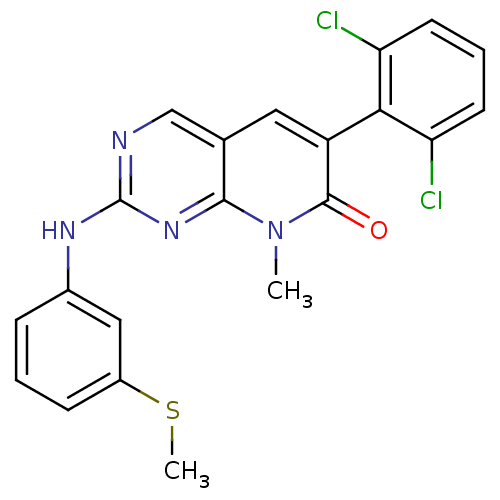
(6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...)Show SMILES CSc1cccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)c1 |(-12.18,1.86,;-10.84,1.09,;-9.51,1.86,;-9.51,3.4,;-8.18,4.17,;-6.84,3.4,;-6.84,1.86,;-5.51,1.08,;-4.18,1.85,;-4.18,3.39,;-2.84,4.16,;-1.51,3.4,;-.18,4.17,;1.16,3.4,;2.49,4.17,;2.49,5.71,;1.16,6.48,;3.83,6.48,;5.16,5.71,;5.16,4.17,;3.83,3.39,;3.83,1.85,;1.16,1.86,;2.49,1.09,;-.18,1.09,;-.18,-.45,;-1.51,1.86,;-2.84,1.08,;-8.18,1.08,)| Show InChI InChI=1S/C21H16Cl2N4OS/c1-27-19-12(9-15(20(27)28)18-16(22)7-4-8-17(18)23)11-24-21(26-19)25-13-5-3-6-14(10-13)29-2/h3-11H,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM31085
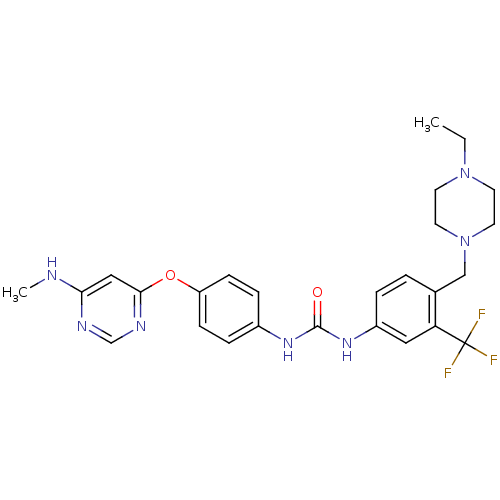
(1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4cc(NC)ncn4)cc3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H30F3N7O2/c1-3-35-10-12-36(13-11-35)16-18-4-5-20(14-22(18)26(27,28)29)34-25(37)33-19-6-8-21(9-7-19)38-24-15-23(30-2)31-17-32-24/h4-9,14-15,17H,3,10-13,16H2,1-2H3,(H,30,31,32)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PCBioAssay
| n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2H993KN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM31085

(1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4cc(NC)ncn4)cc3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H30F3N7O2/c1-3-35-10-12-36(13-11-35)16-18-4-5-20(14-22(18)26(27,28)29)34-25(37)33-19-6-8-21(9-7-19)38-24-15-23(30-2)31-17-32-24/h4-9,14-15,17H,3,10-13,16H2,1-2H3,(H,30,31,32)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM31085

(1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4cc(NC)ncn4)cc3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H30F3N7O2/c1-3-35-10-12-36(13-11-35)16-18-4-5-20(14-22(18)26(27,28)29)34-25(37)33-19-6-8-21(9-7-19)38-24-15-23(30-2)31-17-32-24/h4-9,14-15,17H,3,10-13,16H2,1-2H3,(H,30,31,32)(H2,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50368452
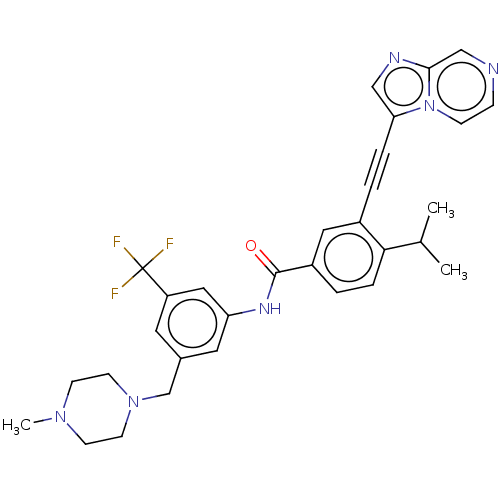
(CHEMBL4168305)Show SMILES CC(C)c1ccc(cc1C#Cc1cnc2cnccn12)C(=O)Nc1cc(CN2CCN(C)CC2)cc(c1)C(F)(F)F Show InChI InChI=1S/C31H31F3N6O/c1-21(2)28-7-5-24(16-23(28)4-6-27-18-36-29-19-35-8-9-40(27)29)30(41)37-26-15-22(14-25(17-26)31(32,33)34)20-39-12-10-38(3)11-13-39/h5,7-9,14-19,21H,10-13,20H2,1-3H3,(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a |
Jinan University
Curated by ChEMBL
| Assay Description
Binding affinity to human EPHB2 |
J Med Chem 61: 7977-7990 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01045
BindingDB Entry DOI: 10.7270/Q24Q7XHN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM21
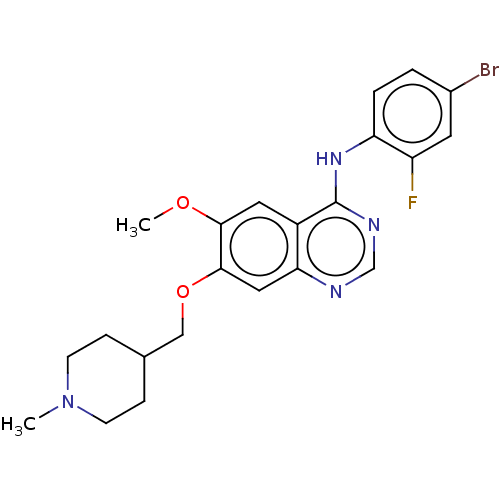
(CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM21

(CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PCBioAssay
| n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2H993KN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2H993KN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM21

(CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM36409
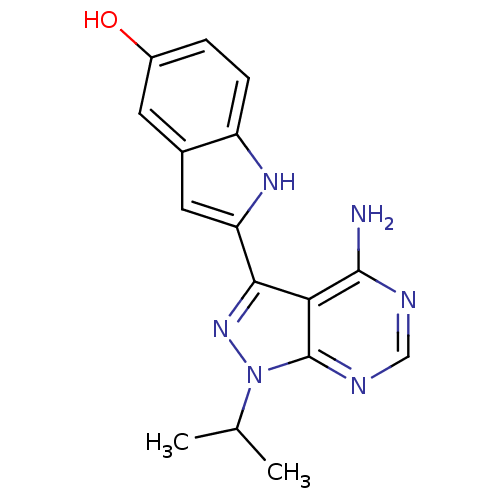
(2-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin...)Show SMILES CC(C)n1nc(-c2cc3cc(O)ccc3[nH]2)c2c(N)ncnc12 Show InChI InChI=1S/C16H16N6O/c1-8(2)22-16-13(15(17)18-7-19-16)14(21-22)12-6-9-5-10(23)3-4-11(9)20-12/h3-8,20,23H,1-2H3,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50237710
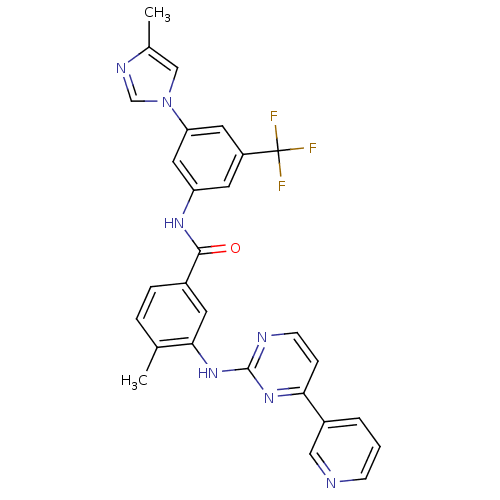
(4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...)Show SMILES Cc1cn(cn1)-c1cc(NC(=O)c2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM60665
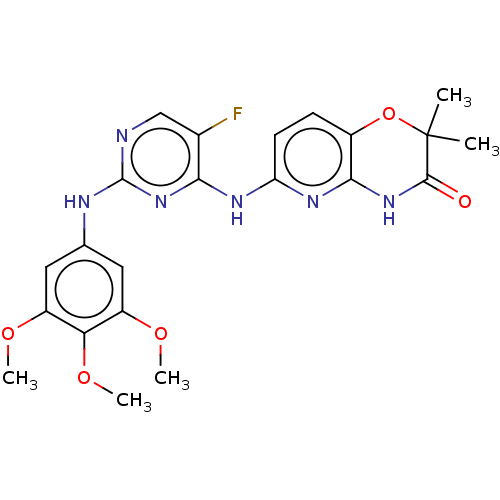
(BDBM50249542 | US9145414, R406 | US9212178, R406)Show SMILES COc1cc(Nc2ncc(F)c(Nc3ccc4OC(C)(C)C(=O)Nc4n3)n2)cc(OC)c1OC Show InChI InChI=1S/C22H23FN6O5/c1-22(2)20(30)28-19-13(34-22)6-7-16(27-19)26-18-12(23)10-24-21(29-18)25-11-8-14(31-3)17(33-5)15(9-11)32-4/h6-10H,1-5H3,(H3,24,25,26,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50505541

(CHEMBL4465866)Show SMILES [H][C@]12C[C@@H](NCCOCCOCCOCCNC(=O)CCC3=[N+]4C(C=C3)=Cc3c(C)cc(C)n3[B-]4(F)F)[C@@H](OC)[C@](C)(O1)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r,c:24,26,t:21| Show InChI InChI=1S/C49H54BF2N7O7/c1-29-25-30(2)58-39(29)26-32-14-13-31(59(32)50(58,51)52)15-16-40(60)54-18-20-64-22-24-65-23-21-63-19-17-53-36-27-41-56-37-11-7-5-9-33(37)43-44-35(28-55-48(44)61)42-34-10-6-8-12-38(34)57(46(42)45(43)56)49(3,66-41)47(36)62-4/h5-14,25-26,36,41,47,53H,15-24,27-28H2,1-4H3,(H,54,60)(H,55,61)/t36-,41-,47-,49+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 985 | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant N-terminal His-FLAG-GST-tagged EPHB2 (unknown origin) (581 to 986 residues) expressed in baculovirus infected Sf9 ins... |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126641
BindingDB Entry DOI: 10.7270/Q2RV0S0K |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM21079
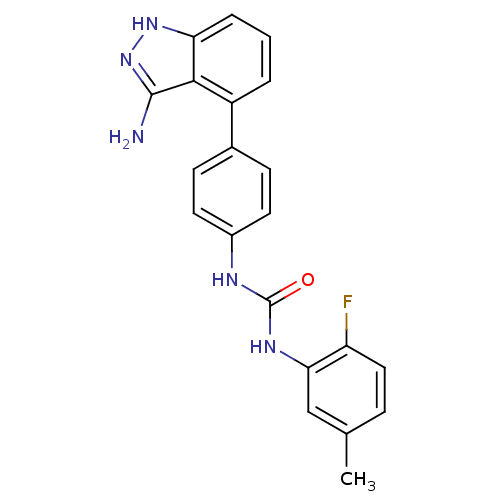
(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM482158
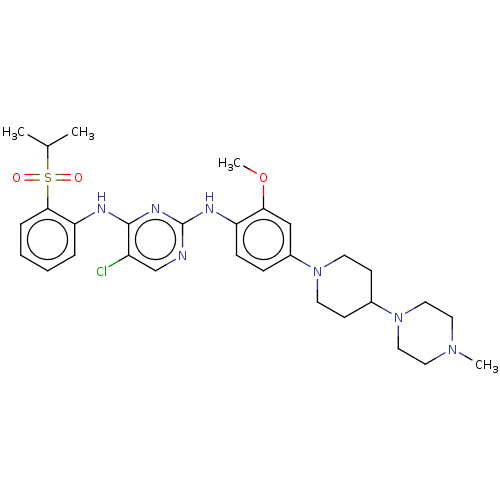
(BDBM50242742 | TAE684)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1)N1CCC(CC1)N1CCN(C)CC1 Show InChI InChI=1S/C30H40ClN7O3S/c1-21(2)42(39,40)28-8-6-5-7-26(28)33-29-24(31)20-32-30(35-29)34-25-10-9-23(19-27(25)41-4)37-13-11-22(12-14-37)38-17-15-36(3)16-18-38/h5-10,19-22H,11-18H2,1-4H3,(H2,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to EPHB2 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2H993KN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM31096
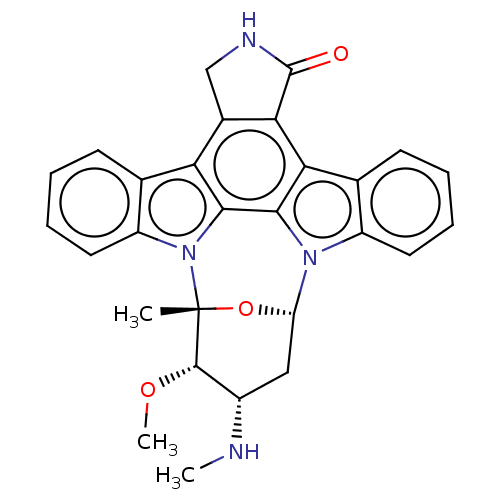
(CHEMBL290084 | Staurosporine | cid_451705)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2H993KN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM4622
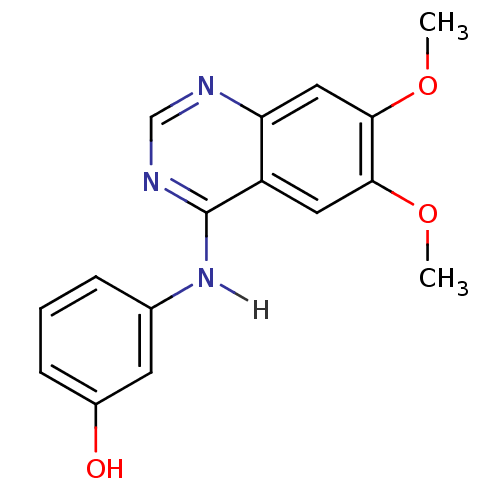
(3-[(6,7-dimethoxyquinazolin-4-yl)amino]phenol | An...)Show InChI InChI=1S/C16H15N3O3/c1-21-14-7-12-13(8-15(14)22-2)17-9-18-16(12)19-10-4-3-5-11(20)6-10/h3-9,20H,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a |
Protana Inc.
Curated by ChEMBL
| Assay Description
Equilibrium binding constant for EPH receptor B2 |
J Med Chem 48: 3221-30 (2005)
Article DOI: 10.1021/jm0492204
BindingDB Entry DOI: 10.7270/Q2DF6QRN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM4779
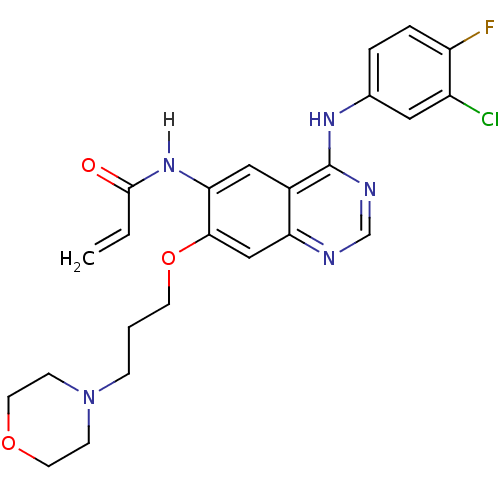
(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2H993KN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50242409
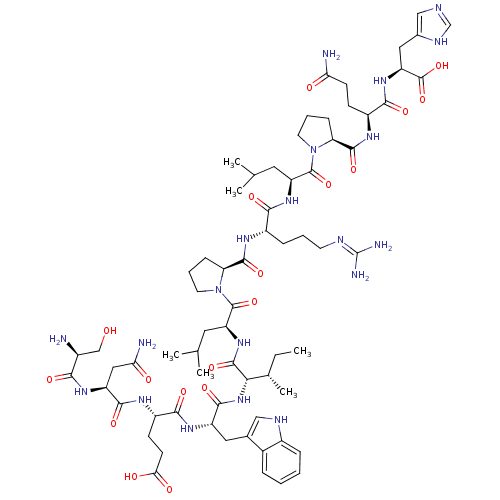
(CHEMBL526325 | SNEWILPRLPQH)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O |r,wU:39.41,22.29,4.4,46.47,57.60,61.63,83.87,96.99,wD:31.37,8.20,2.2,72.74,87.90,(1.44,-23.28,;2.63,-24.25,;4.08,-23.7,;4.32,-22.19,;5.26,-24.69,;5.02,-26.21,;3.58,-26.74,;2.38,-25.77,;3.33,-28.26,;4.52,-29.24,;5.96,-28.69,;6.37,-27.21,;7.9,-27.14,;8.45,-28.58,;9.89,-29.14,;10.12,-30.65,;8.91,-31.61,;7.48,-31.06,;7.24,-29.54,;1.88,-28.81,;1.92,-30.55,;3.08,-31.2,;.55,-31.25,;-.74,-30.39,;-.64,-28.86,;-1.93,-28,;-3.3,-28.7,;-1.84,-26.46,;.46,-32.78,;-.92,-33.47,;-2.2,-32.61,;-1.02,-35,;.27,-35.85,;1.64,-35.17,;1.74,-33.63,;2.92,-36.02,;-2.39,-35.69,;-2.48,-37.22,;-1.2,-38.07,;-3.87,-37.92,;-3.95,-39.45,;-5.16,-37.06,;-6.53,-37.75,;6.71,-24.14,;7.91,-25.11,;6.95,-22.62,;8.39,-22.07,;8.64,-20.55,;7.45,-19.58,;7.7,-18.06,;6.02,-20.13,;9.58,-23.05,;9.34,-24.57,;11.03,-22.51,;11.59,-20.92,;13.27,-20.97,;13.74,-22.57,;12.35,-23.23,;12.29,-24.77,;10.93,-25.5,;13.59,-25.58,;14.99,-24.94,;15.07,-23.4,;16.45,-22.7,;16.51,-21.15,;17.88,-20.45,;17.96,-18.92,;16.67,-18.08,;19.34,-18.22,;16.26,-25.82,;16.11,-27.36,;17.66,-25.19,;18.91,-26.08,;18.87,-27.62,;20.17,-28.43,;20.13,-29.97,;21.53,-27.7,;20.31,-25.44,;21.56,-26.33,;20.45,-23.91,;19.28,-22.7,;20.06,-21.21,;21.71,-21.49,;21.69,-23.03,;23.03,-23.76,;23.08,-25.3,;24.35,-22.95,;25.7,-23.68,;25.75,-25.21,;27.12,-25.95,;27.15,-27.47,;25.86,-28.26,;28.52,-28.21,;27.03,-22.87,;26.98,-21.32,;28.36,-23.59,;29.7,-22.77,;29.64,-21.22,;30.94,-20.43,;32.36,-20.98,;33.34,-19.81,;32.53,-18.5,;31.04,-18.87,;31.03,-23.49,;32.36,-22.68,;31.07,-25.02,)| Show InChI InChI=1S/C68H104N20O18/c1-7-36(6)55(86-61(99)45(27-37-30-76-41-14-9-8-13-39(37)41)82-58(96)44(19-21-54(92)93)78-60(98)46(29-53(71)91)81-56(94)40(69)32-89)64(102)84-48(26-35(4)5)66(104)88-24-11-16-50(88)62(100)79-42(15-10-22-75-68(72)73)57(95)83-47(25-34(2)3)65(103)87-23-12-17-51(87)63(101)80-43(18-20-52(70)90)59(97)85-49(67(105)106)28-38-31-74-33-77-38/h8-9,13-14,30-31,33-36,40,42-51,55,76,89H,7,10-12,15-29,32,69H2,1-6H3,(H2,70,90)(H2,71,91)(H,74,77)(H,78,98)(H,79,100)(H,80,101)(H,81,94)(H,82,96)(H,83,95)(H,84,102)(H,85,97)(H,86,99)(H,92,93)(H,105,106)(H4,72,73,75)/t36-,40-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,55-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human EphB2 receptor by isothermal titration calorimetry |
J Biol Chem 282: 36505-13 (2007)
Article DOI: 10.1074/jbc.M706340200
BindingDB Entry DOI: 10.7270/Q29C6X7S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50355499
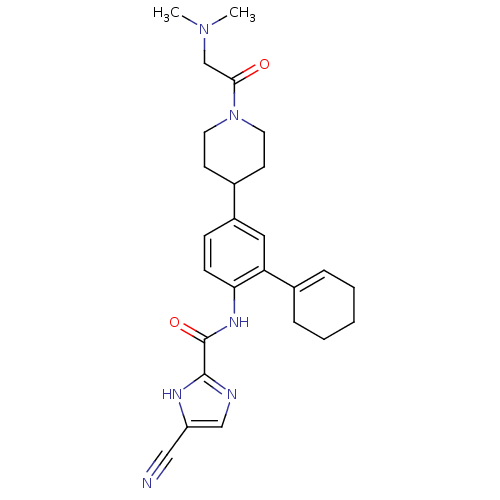
(CHEMBL1908395 | CHEMBL1908842)Show SMILES CN(C)CC(=O)N1CCC(CC1)c1ccc(NC(=O)c2ncc([nH]2)C#N)c(c1)C1=CCCCC1 |t:31| Show InChI InChI=1S/C26H32N6O2/c1-31(2)17-24(33)32-12-10-18(11-13-32)20-8-9-23(22(14-20)19-6-4-3-5-7-19)30-26(34)25-28-16-21(15-27)29-25/h6,8-9,14,16,18H,3-5,7,10-13,17H2,1-2H3,(H,28,29)(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50332294
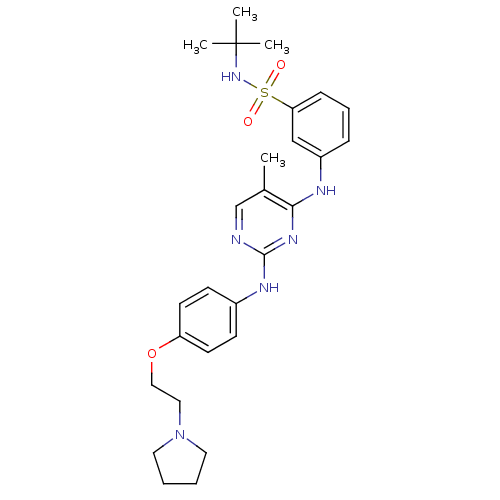
(CHEMBL1287853 | N-tert-butyl-3-(5-methyl-2-(4-(2-(...)Show SMILES Cc1cnc(Nc2ccc(OCCN3CCCC3)cc2)nc1Nc1cccc(c1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C27H36N6O3S/c1-20-19-28-26(30-21-10-12-23(13-11-21)36-17-16-33-14-5-6-15-33)31-25(20)29-22-8-7-9-24(18-22)37(34,35)32-27(2,3)4/h7-13,18-19,32H,5-6,14-17H2,1-4H3,(H2,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50242406
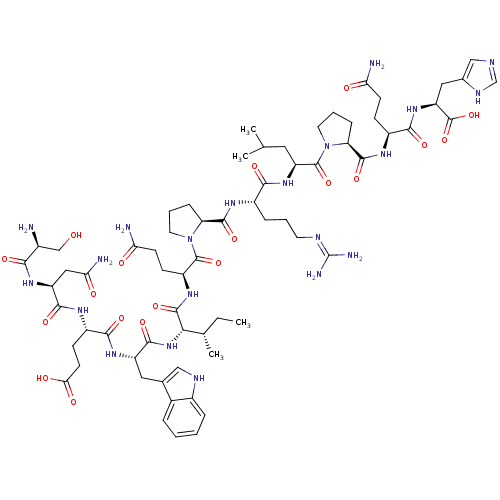
(CHEMBL448652 | SNEWIQPRLPQH)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O |r,wU:22.29,4.4,73.75,88.91,wD:39.41,31.37,8.20,2.2,46.47,58.61,62.64,84.88,97.100,(6.11,-.72,;6.16,-2.26,;7.52,-2.99,;8.83,-2.19,;7.56,-4.54,;6.25,-5.35,;4.9,-4.62,;4.85,-3.07,;3.59,-5.43,;3.63,-6.97,;4.99,-7.7,;6.37,-7.03,;7.44,-8.15,;6.7,-9.49,;7.23,-10.95,;6.23,-12.12,;4.71,-11.85,;4.2,-10.39,;5.19,-9.22,;2.23,-4.7,;.91,-5.5,;.96,-7.04,;-.45,-4.77,;-.49,-3.23,;.82,-2.42,;.78,-.88,;-.58,-.15,;2.09,-.07,;-1.75,-5.58,;-3.11,-4.85,;-3.15,-3.31,;-4.42,-5.66,;-4.38,-7.2,;-3.02,-7.93,;-1.7,-7.12,;-2.97,-9.47,;-5.78,-4.92,;-7.09,-5.74,;-7.05,-7.27,;-8.44,-5,;-8.49,-3.47,;-9.75,-5.81,;-11.11,-5.08,;8.92,-5.27,;8.96,-6.81,;10.23,-4.46,;11.59,-5.19,;11.64,-6.73,;10.32,-7.54,;10.37,-9.08,;11.72,-9.81,;9.05,-9.89,;12.9,-4.38,;12.85,-2.84,;14.25,-5.11,;14.6,-6.76,;16.28,-6.94,;16.97,-5.41,;15.65,-4.56,;15.81,-3.04,;14.57,-2.14,;17.22,-2.4,;18.52,-3.23,;18.46,-4.76,;19.76,-5.59,;19.7,-7.12,;21.01,-7.95,;20.95,-9.49,;19.59,-10.2,;22.25,-10.31,;19.89,-2.51,;19.94,-.96,;21.19,-3.33,;22.55,-2.62,;22.62,-1.07,;23.98,-.35,;24.04,1.18,;25.28,-1.18,;23.85,-3.43,;25.22,-2.71,;23.8,-4.98,;22.47,-6.01,;23.05,-7.6,;24.72,-7.54,;24.9,-6.01,;26.35,-5.46,;26.59,-3.94,;27.53,-6.44,;28.97,-5.9,;29.22,-4.39,;30.68,-3.83,;30.92,-2.33,;29.74,-1.36,;32.37,-1.78,;30.18,-6.88,;29.92,-8.42,;31.6,-6.34,;32.81,-7.32,;32.56,-8.86,;33.74,-9.82,;33.63,-11.38,;35.06,-11.95,;36.04,-10.76,;35.22,-9.46,;34.23,-6.78,;35.44,-7.77,;34.48,-5.28,)| Show InChI InChI=1S/C67H101N21O19/c1-5-34(4)54(86-60(100)44(26-35-29-76-39-12-7-6-11-37(35)39)83-57(97)42(18-21-53(93)94)78-59(99)45(28-52(71)92)82-55(95)38(68)31-89)63(103)81-43(17-20-51(70)91)64(104)87-23-9-14-48(87)61(101)79-40(13-8-22-75-67(72)73)56(96)84-46(25-33(2)3)65(105)88-24-10-15-49(88)62(102)80-41(16-19-50(69)90)58(98)85-47(66(106)107)27-36-30-74-32-77-36/h6-7,11-12,29-30,32-34,38,40-49,54,76,89H,5,8-10,13-28,31,68H2,1-4H3,(H2,69,90)(H2,70,91)(H2,71,92)(H,74,77)(H,78,99)(H,79,101)(H,80,102)(H,81,103)(H,82,95)(H,83,97)(H,84,96)(H,85,98)(H,86,100)(H,93,94)(H,106,107)(H4,72,73,75)/t34-,38-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,54-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human EphB2 receptor by isothermal titration calorimetry |
J Biol Chem 282: 36505-13 (2007)
Article DOI: 10.1074/jbc.M706340200
BindingDB Entry DOI: 10.7270/Q29C6X7S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50242410

(CHEMBL508644 | SNEWIQPKLPQH)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O |r| Show InChI InChI=1S/C67H101N19O19/c1-5-35(4)55(84-61(98)45(27-36-30-74-40-13-7-6-12-38(36)40)81-58(95)43(19-22-54(91)92)76-60(97)46(29-53(72)90)80-56(93)39(69)32-87)64(101)79-44(18-21-52(71)89)65(102)85-24-10-15-49(85)62(99)77-41(14-8-9-23-68)57(94)82-47(26-34(2)3)66(103)86-25-11-16-50(86)63(100)78-42(17-20-51(70)88)59(96)83-48(67(104)105)28-37-31-73-33-75-37/h6-7,12-13,30-31,33-35,39,41-50,55,74,87H,5,8-11,14-29,32,68-69H2,1-4H3,(H2,70,88)(H2,71,89)(H2,72,90)(H,73,75)(H,76,97)(H,77,99)(H,78,100)(H,79,101)(H,80,93)(H,81,95)(H,82,94)(H,83,96)(H,84,98)(H,91,92)(H,104,105)/t35-,39-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,55-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human EphB2 receptor by isothermal titration calorimetry |
J Biol Chem 282: 36505-13 (2007)
Article DOI: 10.1074/jbc.M706340200
BindingDB Entry DOI: 10.7270/Q29C6X7S |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM4621

(Anilinoquinazoline deriv. 4 | CHEMBL150315 | N-(4-...)Show InChI InChI=1S/C16H13ClFN3O2/c1-22-14-6-10-13(7-15(14)23-2)19-8-20-16(10)21-12-4-3-9(17)5-11(12)18/h3-8H,1-2H3,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a |
Protana Inc.
Curated by ChEMBL
| Assay Description
Equilibrium binding constant for EPH receptor B2 |
J Med Chem 48: 3221-30 (2005)
Article DOI: 10.1021/jm0492204
BindingDB Entry DOI: 10.7270/Q2DF6QRN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM13336
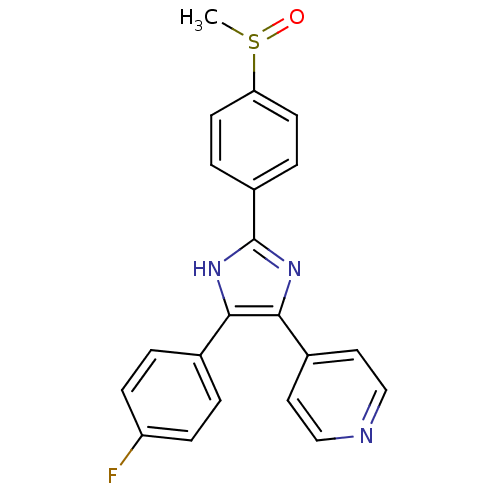
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a |
Protana Inc.
Curated by ChEMBL
| Assay Description
Equilibrium binding constant for EPH receptor B2 |
J Med Chem 48: 3221-30 (2005)
Article DOI: 10.1021/jm0492204
BindingDB Entry DOI: 10.7270/Q2DF6QRN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50308060
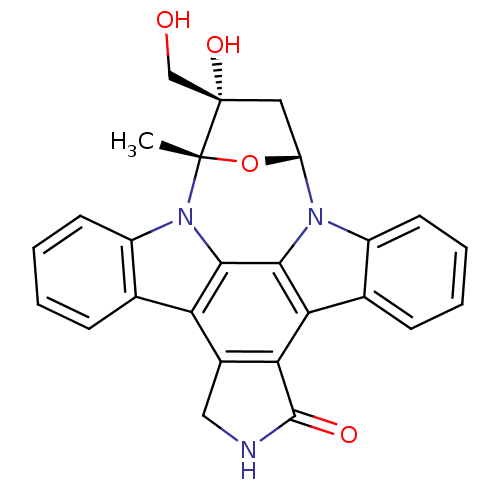
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM3032
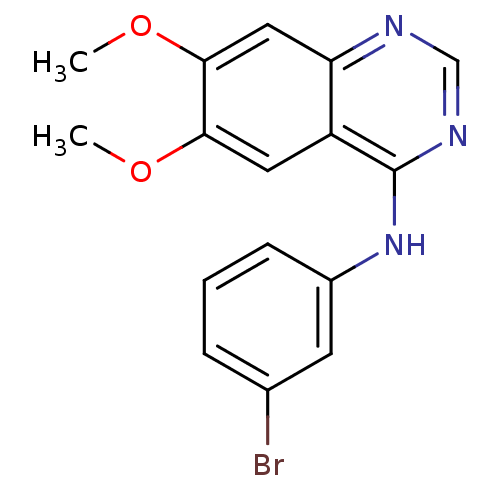
(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a |
Protana Inc.
Curated by ChEMBL
| Assay Description
Equilibrium binding constant for EPH receptor B2 |
J Med Chem 48: 3221-30 (2005)
Article DOI: 10.1021/jm0492204
BindingDB Entry DOI: 10.7270/Q2DF6QRN |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM24654
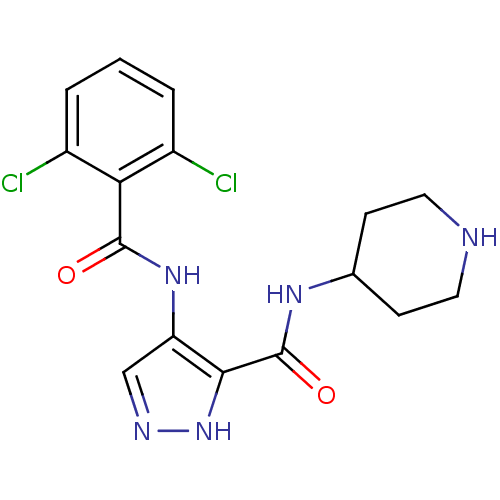
(4-N-(2,6-dichlorobenzene)-3-N-(piperidin-4-yl)-1H-...)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1cn[nH]c1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM26474
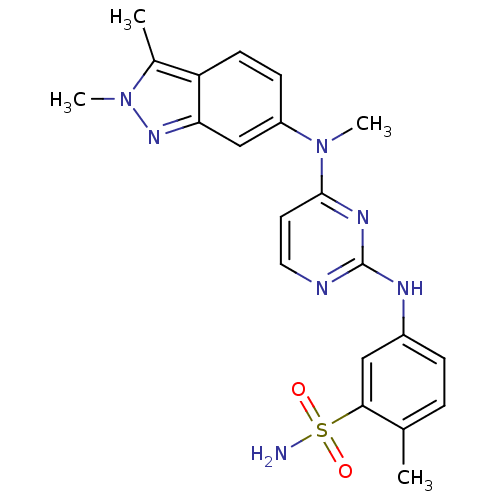
(5-({4-[(2,3-dimethyl-2H-indazol-6-yl)(methyl)amino...)Show SMILES CN(c1ccc2c(C)n(C)nc2c1)c1ccnc(Nc2ccc(C)c(c2)S(N)(=O)=O)n1 Show InChI InChI=1S/C21H23N7O2S/c1-13-5-6-15(11-19(13)31(22,29)30)24-21-23-10-9-20(25-21)27(3)16-7-8-17-14(2)28(4)26-18(17)12-16/h5-12H,1-4H3,(H2,22,29,30)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50184807
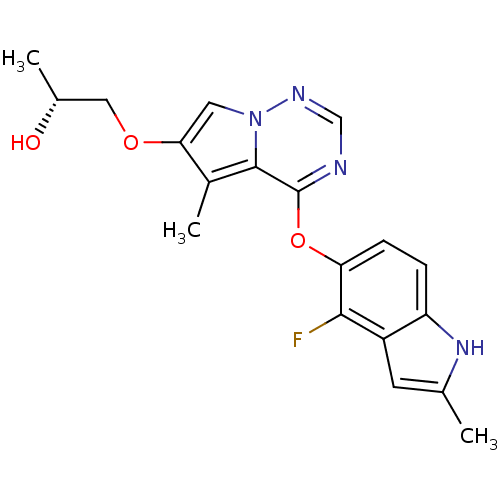
((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-me...)Show SMILES C[C@@H](O)COc1cn2ncnc(Oc3ccc4[nH]c(C)cc4c3F)c2c1C Show InChI InChI=1S/C19H19FN4O3/c1-10-6-13-14(23-10)4-5-15(17(13)20)27-19-18-12(3)16(26-8-11(2)25)7-24(18)22-9-21-19/h4-7,9,11,23,25H,8H2,1-3H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50355497
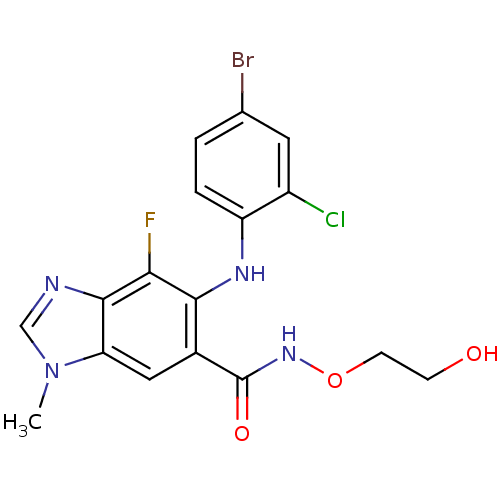
(AZD-6244 | CHEMBL1614701)Show SMILES Cn1cnc2c(F)c(Nc3ccc(Br)cc3Cl)c(cc12)C(=O)NOCCO Show InChI InChI=1S/C17H15BrClFN4O3/c1-24-8-21-16-13(24)7-10(17(26)23-27-5-4-25)15(14(16)20)22-12-3-2-9(18)6-11(12)19/h2-3,6-8,22,25H,4-5H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for EPHB2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data