 Found 34 hits of kd data for polymerid = 50001641
Found 34 hits of kd data for polymerid = 50001641 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heparanase
(Homo sapiens (Human)) | BDBM50378647
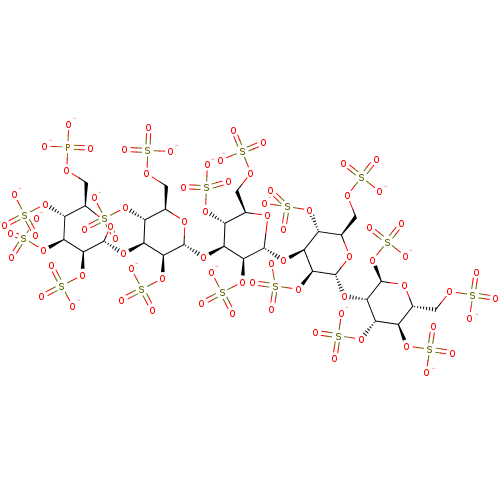
(CHEMBL1627122 | PI-88)Show SMILES [O-]P([O-])(=O)OC[C@H]1O[C@H](O[C@H]2[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]3[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@H]4[C@H](OS([O-])(=O)=O)[C@@H](COS([O-])(=O)=O)O[C@H](O[C@@H]5[C@@H](OS([O-])(=O)=O)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@H]4OS([O-])(=O)=O)[C@H]3OS([O-])(=O)=O)[C@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C30H53O77PS16/c31-108(32,33)82-1-6-14(99-116(55,56)57)20(102-119(64,65)66)25(106-123(76,77)78)29(87-6)94-17-12(97-114(49,50)51)8(3-84-110(37,38)39)89-27(23(17)104-121(70,71)72)92-16-11(96-113(46,47)48)7(2-83-109(34,35)36)88-26(22(16)103-120(67,68)69)93-18-13(98-115(52,53)54)9(4-85-111(40,41)42)90-28(24(18)105-122(73,74)75)95-21-19(101-118(61,62)63)15(100-117(58,59)60)10(5-86-112(43,44)45)91-30(21)107-124(79,80)81/h6-30H,1-5H2,(H2,31,32,33)(H,34,35,36)(H,37,38,39)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)/p-18/t6-,7-,8-,9-,10-,11-,12-,13-,14-,15-,16+,17+,18+,19+,20+,21+,22+,23+,24+,25+,26-,27-,28-,29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307395

(12-(4-Phenyl[1,2,3]triazol-1-yl)dodecyl 2,3,4,6-Te...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2[C@@H](OCCCCCCCCCCCCn3cc(nn3)-c3ccccc3)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C50H81N3O74S16/c54-128(55,56)103-19-26-31(117-133(69,70)71)36(113-48-43(125-141(93,94)95)37(32(118-134(72,73)74)27(110-48)20-104-129(57,58)59)115-50-45(127-143(99,100)101)40(123-139(87,88)89)35(121-137(81,82)83)30(112-50)23-107-132(66,67)68)42(124-140(90,91)92)47(109-26)114-38-33(119-135(75,76)77)28(21-105-130(60,61)62)111-49(44(38)126-142(96,97)98)116-41-39(122-138(84,85)86)34(120-136(78,79)80)29(22-106-131(63,64)65)108-46(41)102-17-13-8-6-4-2-1-3-5-7-12-16-53-18-25(51-52-53)24-14-10-9-11-15-24/h9-11,14-15,18,26-50H,1-8,12-13,16-17,19-23H2,(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)(H,87,88,89)(H,90,91,92)(H,93,94,95)(H,96,97,98)(H,99,100,101)/p-16/t26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36+,37+,38+,39+,40+,41+,42+,43+,44+,45+,46+,47-,48-,49-,50-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307396
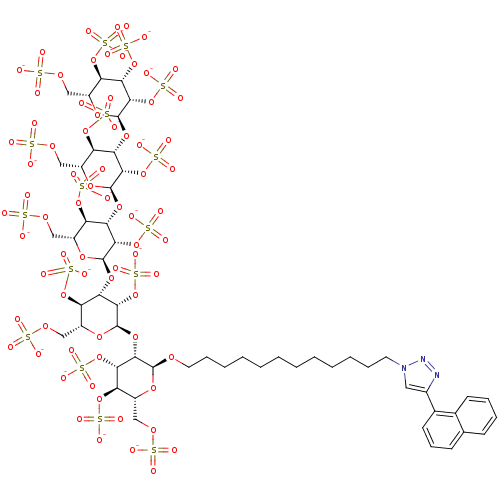
(12-(4-Naphthalen-1-yl[1,2,3]triazol-1-yl)dodecyl 2...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCCCCCCCCCCCn2cc(nn2)-c2cccc3ccccc23)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C54H83N3O74S16/c58-132(59,60)107-21-30-35(121-137(73,74)75)40(117-52-47(129-145(97,98)99)41(36(122-138(76,77)78)31(114-52)22-108-133(61,62)63)119-54-49(131-147(103,104)105)44(127-143(91,92)93)39(125-141(85,86)87)34(116-54)25-111-136(70,71)72)46(128-144(94,95)96)51(113-30)118-42-37(123-139(79,80)81)32(23-109-134(64,65)66)115-53(48(42)130-146(100,101)102)120-45-43(126-142(88,89)90)38(124-140(82,83)84)33(24-110-135(67,68)69)112-50(45)106-19-12-8-6-4-2-1-3-5-7-11-18-57-20-29(55-56-57)28-17-13-15-26-14-9-10-16-27(26)28/h9-10,13-17,20,30-54H,1-8,11-12,18-19,21-25H2,(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)(H,91,92,93)(H,94,95,96)(H,97,98,99)(H,100,101,102)(H,103,104,105)/p-16/t30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40+,41+,42+,43+,44+,45+,46+,47+,48+,49+,50+,51-,52-,53-,54-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307390
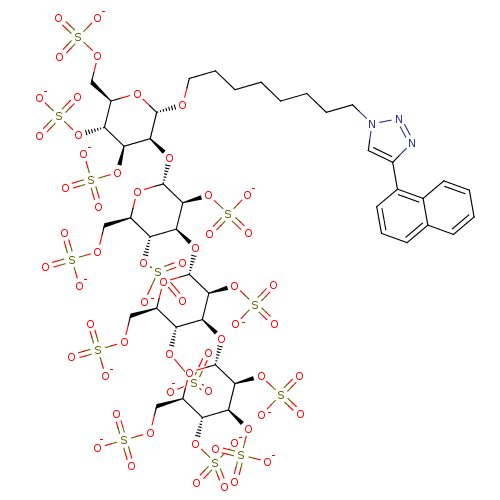
(8-(4-Naphthalen-1-yl[1,2,3]triazol-1-yl)octyl 2,3,...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCCCCCCCn2cc(nn2)-c2cccc3ccccc23)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C44H65N3O60S13/c48-108(49,50)88-17-25-29(99-112(60,61)62)33(97-44-40(107-120(84,85)86)36(104-117(75,76)77)32(102-115(69,70)71)28(95-44)20-91-111(57,58)59)38(105-118(78,79)80)42(93-25)96-34-30(100-113(63,64)65)26(18-89-109(51,52)53)94-43(39(34)106-119(81,82)83)98-37-35(103-116(72,73)74)31(101-114(66,67)68)27(19-90-110(54,55)56)92-41(37)87-15-8-4-2-1-3-7-14-47-16-24(45-46-47)23-13-9-11-21-10-5-6-12-22(21)23/h5-6,9-13,16,25-44H,1-4,7-8,14-15,17-20H2,(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)/p-13/t25-,26-,27-,28-,29-,30-,31-,32-,33+,34+,35+,36+,37+,38+,39+,40+,41+,42-,43-,44-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307386

(CHEMBL604367 | Dodecyl 2,3,4,6-Tetra-O-sulfo-alpha...)Show SMILES CCCCCCCCCCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C42H76O74S16/c1-2-3-4-5-6-7-8-9-10-11-12-91-38-33(31(111-127(73,74)75)26(109-125(67,68)69)21(97-38)16-95-120(52,53)54)105-41-36(115-131(85,86)87)30(25(108-124(64,65)66)20(100-41)15-94-119(49,50)51)103-39-34(113-129(79,80)81)28(23(106-122(58,59)60)18(98-39)13-92-117(43,44)45)102-40-35(114-130(82,83)84)29(24(107-123(61,62)63)19(99-40)14-93-118(46,47)48)104-42-37(116-132(88,89)90)32(112-128(76,77)78)27(110-126(70,71)72)22(101-42)17-96-121(55,56)57/h18-42H,2-17H2,1H3,(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)(H,88,89,90)/p-16/t18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28+,29+,30+,31+,32+,33+,34+,35+,36+,37+,38+,39-,40-,41-,42-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307399
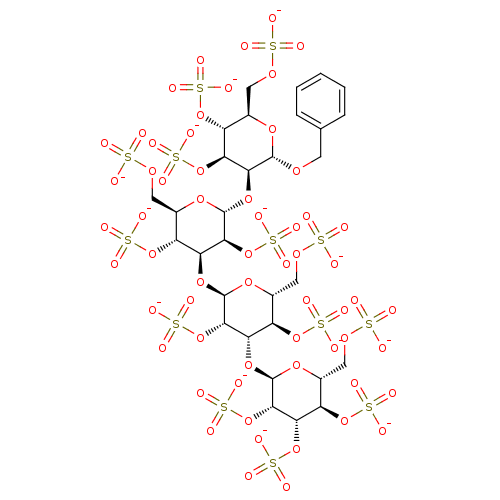
(Benzyl 2,3,4,6-Tetra-O-sulfo-alpha-D-mannopyranosy...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCc2ccccc2)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C31H48O60S13/c32-92(33,34)72-7-12-16(83-96(44,45)46)20(81-31-27(91-104(68,69)70)23(88-101(59,60)61)19(86-99(53,54)55)15(79-31)10-75-95(41,42)43)25(89-102(62,63)64)29(77-12)80-21-17(84-97(47,48)49)13(8-73-93(35,36)37)78-30(26(21)90-103(65,66)67)82-24-22(87-100(56,57)58)18(85-98(50,51)52)14(9-74-94(38,39)40)76-28(24)71-6-11-4-2-1-3-5-11/h1-5,12-31H,6-10H2,(H,32,33,34)(H,35,36,37)(H,38,39,40)(H,41,42,43)(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)/p-13/t12-,13-,14-,15-,16-,17-,18-,19-,20+,21+,22+,23+,24+,25+,26+,27+,28+,29-,30-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307402
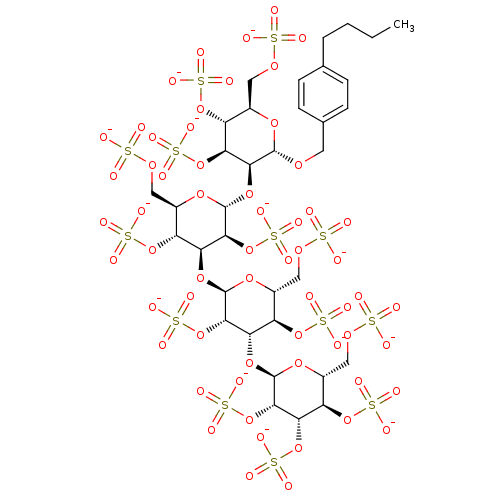
(4-Butylbenzyl 2,3,4,6-Tetra-O-sulfo-alpha-D-mannop...)Show SMILES CCCCc1ccc(CO[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)cc1 |r| Show InChI InChI=1S/C35H56O60S13/c1-2-3-4-14-5-7-15(8-6-14)9-75-32-28(26(91-104(60,61)62)22(89-102(54,55)56)18(80-32)12-78-98(42,43)44)86-34-30(94-107(69,70)71)25(21(88-101(51,52)53)17(82-34)11-77-97(39,40)41)84-33-29(93-106(66,67)68)24(20(87-100(48,49)50)16(81-33)10-76-96(36,37)38)85-35-31(95-108(72,73)74)27(92-105(63,64)65)23(90-103(57,58)59)19(83-35)13-79-99(45,46)47/h5-8,16-35H,2-4,9-13H2,1H3,(H,36,37,38)(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)/p-13/t16-,17-,18-,19-,20-,21-,22-,23-,24+,25+,26+,27+,28+,29+,30+,31+,32+,33-,34-,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307388
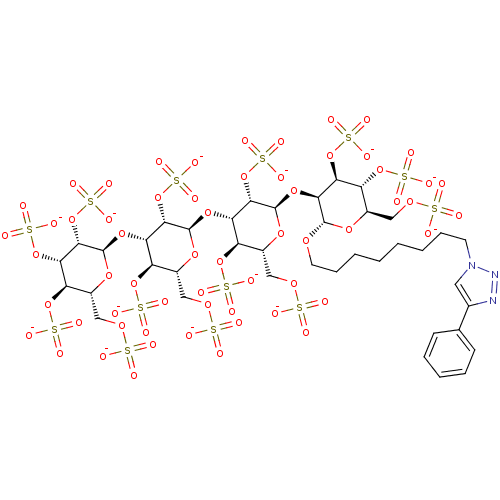
(8-(4-Phenyl[1,2,3]triazol-1-yl)octyl 2,3,4,6-Tetra...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2[C@@H](OCCCCCCCCn3cc(nn3)-c3ccccc3)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C40H63N3O60S13/c44-104(45,46)84-15-21-25(95-108(56,57)58)29(93-40-36(103-116(80,81)82)32(100-113(71,72)73)28(98-111(65,66)67)24(91-40)18-87-107(53,54)55)34(101-114(74,75)76)38(89-21)92-30-26(96-109(59,60)61)22(16-85-105(47,48)49)90-39(35(30)102-115(77,78)79)94-33-31(99-112(68,69)70)27(97-110(62,63)64)23(17-86-106(50,51)52)88-37(33)83-13-9-4-2-1-3-8-12-43-14-20(41-42-43)19-10-6-5-7-11-19/h5-7,10-11,14,21-40H,1-4,8-9,12-13,15-18H2,(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)(H,71,72,73)(H,74,75,76)(H,77,78,79)(H,80,81,82)/p-13/t21-,22-,23-,24-,25-,26-,27-,28-,29+,30+,31+,32+,33+,34+,35+,36+,37+,38-,39-,40-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307391
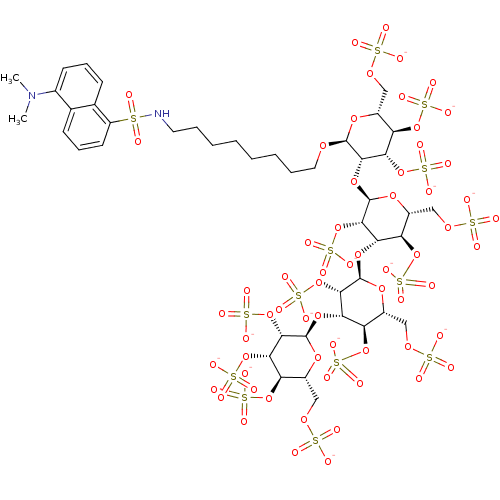
(8-(5-Dimethylaminonaphthalene-1-sulfonamido)octyl ...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCCCCCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C44H70N2O62S14/c1-46(2)23-13-9-12-22-21(23)11-10-14-28(22)109(47,48)45-15-7-5-3-4-6-8-16-88-41-37(35(104-118(73,74)75)31(102-116(67,68)69)26(93-41)19-91-112(55,56)57)99-43-39(107-121(82,83)84)34(30(101-115(64,65)66)25(95-43)18-90-111(52,53)54)97-42-38(106-120(79,80)81)33(29(100-114(61,62)63)24(94-42)17-89-110(49,50)51)98-44-40(108-122(85,86)87)36(105-119(76,77)78)32(103-117(70,71)72)27(96-44)20-92-113(58,59)60/h9-14,24-27,29-45H,3-8,15-20H2,1-2H3,(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60)(H,61,62,63)(H,64,65,66)(H,67,68,69)(H,70,71,72)(H,73,74,75)(H,76,77,78)(H,79,80,81)(H,82,83,84)(H,85,86,87)/p-13/t24-,25-,26-,27-,29-,30-,31-,32-,33+,34+,35+,36+,37+,38+,39+,40+,41+,42-,43-,44-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307401
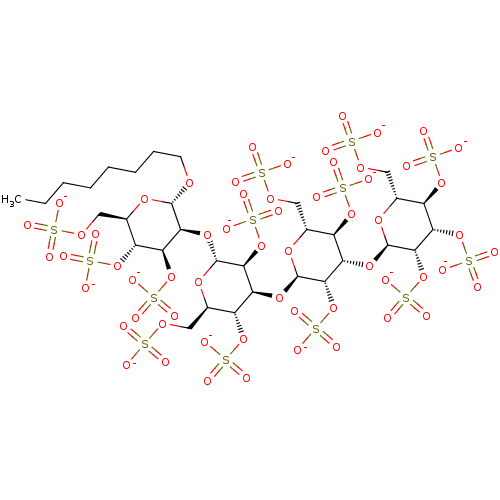
(CHEMBL602113 | Octyl 2,3,4,6-Tetra-O-sulfo-alpha-D...)Show SMILES CCCCCCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C32H58O60S13/c1-2-3-4-5-6-7-8-72-29-25(23(88-101(57,58)59)19(86-99(51,52)53)15(77-29)11-75-95(39,40)41)83-31-27(91-104(66,67)68)22(18(85-98(48,49)50)14(79-31)10-74-94(36,37)38)81-30-26(90-103(63,64)65)21(17(84-97(45,46)47)13(78-30)9-73-93(33,34)35)82-32-28(92-105(69,70)71)24(89-102(60,61)62)20(87-100(54,55)56)16(80-32)12-76-96(42,43)44/h13-32H,2-12H2,1H3,(H,33,34,35)(H,36,37,38)(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)/p-13/t13-,14-,15-,16-,17-,18-,19-,20-,21+,22+,23+,24+,25+,26+,27+,28+,29+,30-,31-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307392
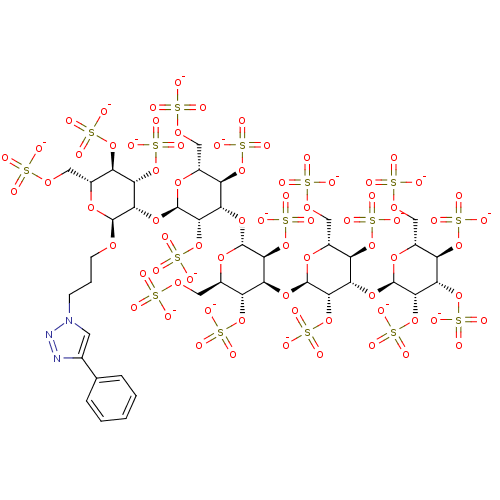
(3-(4-Phenyl[1,2,3]triazol-1-yl)propyl 2,3,4,6-Tetr...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCCn2cc(nn2)-c2ccccc2)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C41H63N3O74S16/c45-119(46,47)94-10-17-22(108-124(60,61)62)27(104-39-34(116-132(84,85)86)28(23(109-125(63,64)65)18(101-39)11-95-120(48,49)50)106-41-36(118-134(90,91)92)31(114-130(78,79)80)26(112-128(72,73)74)21(103-41)14-98-123(57,58)59)33(115-131(81,82)83)38(100-17)105-29-24(110-126(66,67)68)19(12-96-121(51,52)53)102-40(35(29)117-133(87,88)89)107-32-30(113-129(75,76)77)25(111-127(69,70)71)20(13-97-122(54,55)56)99-37(32)93-8-4-7-44-9-16(42-43-44)15-5-2-1-3-6-15/h1-3,5-6,9,17-41H,4,7-8,10-14H2,(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)(H,87,88,89)(H,90,91,92)/p-16/t17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27+,28+,29+,30+,31+,32+,33+,34+,35+,36+,37+,38-,39-,40-,41-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307400

(CHEMBL604366 | hexadecasodium [3-({4-[(4-{[3,5-bis...)Show SMILES CCCCCCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C38H68O74S16/c1-2-3-4-5-6-7-8-87-34-29(27(107-123(69,70)71)22(105-121(63,64)65)17(93-34)12-91-116(48,49)50)101-37-32(111-127(81,82)83)26(21(104-120(60,61)62)16(96-37)11-90-115(45,46)47)99-35-30(109-125(75,76)77)24(19(102-118(54,55)56)14(94-35)9-88-113(39,40)41)98-36-31(110-126(78,79)80)25(20(103-119(57,58)59)15(95-36)10-89-114(42,43)44)100-38-33(112-128(84,85)86)28(108-124(72,73)74)23(106-122(66,67)68)18(97-38)13-92-117(51,52)53/h14-38H,2-13H2,1H3,(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)/p-16/t14-,15-,16-,17-,18-,19-,20-,21-,22-,23-,24+,25+,26+,27+,28+,29+,30+,31+,32+,33+,34+,35-,36-,37-,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307389
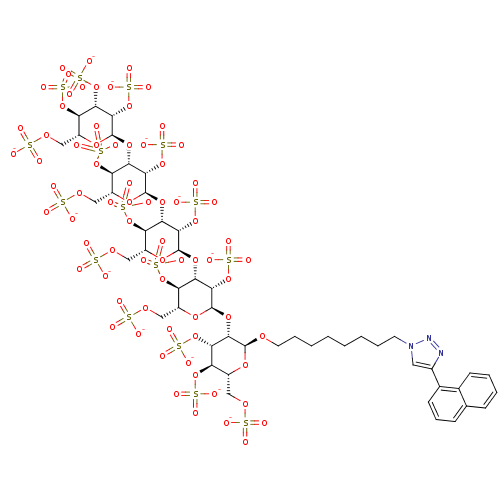
(2,3,4,6-Tetra-O-sulfo-alpha-D-mannopyranosyl-(1->3...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCCCCCCCn2cc(nn2)-c2cccc3ccccc23)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C50H75N3O74S16/c54-128(55,56)103-17-26-31(117-133(69,70)71)36(113-48-43(125-141(93,94)95)37(32(118-134(72,73)74)27(110-48)18-104-129(57,58)59)115-50-45(127-143(99,100)101)40(123-139(87,88)89)35(121-137(81,82)83)30(112-50)21-107-132(66,67)68)42(124-140(90,91)92)47(109-26)114-38-33(119-135(75,76)77)28(19-105-130(60,61)62)111-49(44(38)126-142(96,97)98)116-41-39(122-138(84,85)86)34(120-136(78,79)80)29(20-106-131(63,64)65)108-46(41)102-15-8-4-2-1-3-7-14-53-16-25(51-52-53)24-13-9-11-22-10-5-6-12-23(22)24/h5-6,9-13,16,26-50H,1-4,7-8,14-15,17-21H2,(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)(H,81,82,83)(H,84,85,86)(H,87,88,89)(H,90,91,92)(H,93,94,95)(H,96,97,98)(H,99,100,101)/p-16/t26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36+,37+,38+,39+,40+,41+,42+,43+,44+,45+,46+,47-,48-,49-,50-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307398
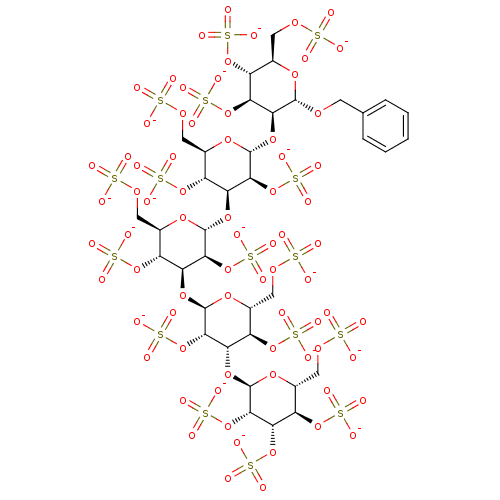
(CHEMBL603122 | Hexadecasodium [2-(benzyloxy)-3-({4...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCc2ccccc2)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]5O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]5OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C37H58O74S16/c38-112(39,40)87-7-13-18(101-117(53,54)55)23(97-35-30(109-125(77,78)79)24(19(102-118(56,57)58)14(94-35)8-88-113(41,42)43)99-37-32(111-127(83,84)85)27(107-123(71,72)73)22(105-121(65,66)67)17(96-37)11-91-116(50,51)52)29(108-124(74,75)76)34(93-13)98-25-20(103-119(59,60)61)15(9-89-114(44,45)46)95-36(31(25)110-126(80,81)82)100-28-26(106-122(68,69)70)21(104-120(62,63)64)16(10-90-115(47,48)49)92-33(28)86-6-12-4-2-1-3-5-12/h1-5,13-37H,6-11H2,(H,38,39,40)(H,41,42,43)(H,44,45,46)(H,47,48,49)(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)(H,71,72,73)(H,74,75,76)(H,77,78,79)(H,80,81,82)(H,83,84,85)/p-16/t13-,14-,15-,16-,17-,18-,19-,20-,21-,22-,23+,24+,25+,26+,27+,28+,29+,30+,31+,32+,33+,34-,35-,36-,37-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307387
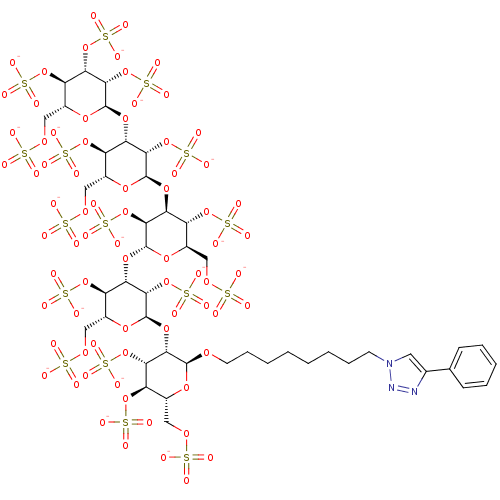
(8-(4-Phenyl[1,2,3]triazol-1-yl)octyl 2,3,4,6-Tetra...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](O[C@@H]2[C@@H](OCCCCCCCCn3cc(nn3)-c3ccccc3)O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C46H73N3O74S16/c50-124(51,52)99-15-22-27(113-129(65,66)67)32(109-44-39(121-137(89,90)91)33(28(114-130(68,69)70)23(106-44)16-100-125(53,54)55)111-46-41(123-139(95,96)97)36(119-135(83,84)85)31(117-133(77,78)79)26(108-46)19-103-128(62,63)64)38(120-136(86,87)88)43(105-22)110-34-29(115-131(71,72)73)24(17-101-126(56,57)58)107-45(40(34)122-138(92,93)94)112-37-35(118-134(80,81)82)30(116-132(74,75)76)25(18-102-127(59,60)61)104-42(37)98-13-9-4-2-1-3-8-12-49-14-21(47-48-49)20-10-6-5-7-11-20/h5-7,10-11,14,22-46H,1-4,8-9,12-13,15-19H2,(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)(H,71,72,73)(H,74,75,76)(H,77,78,79)(H,80,81,82)(H,83,84,85)(H,86,87,88)(H,89,90,91)(H,92,93,94)(H,95,96,97)/p-16/t22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32+,33+,34+,35+,36+,37+,38+,39+,40+,41+,42+,43-,44-,45-,46-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307394
(3-(5-Dimethylaminonaphthalene-1-sulfonamido)propyl...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCCO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]1O[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C45H70N2O76S17/c1-47(2)19-8-3-7-18-17(19)6-4-9-25(18)124(48,49)46-10-5-11-98-41-36(34(118-135(80,81)82)29(116-133(74,75)76)23(104-41)15-102-128(59,60)61)112-44-39(122-139(92,93)94)33(28(115-132(71,72)73)22(107-44)14-101-127(56,57)58)110-42-37(120-137(86,87)88)31(26(113-130(65,66)67)20(105-42)12-99-125(50,51)52)109-43-38(121-138(89,90)91)32(27(114-131(68,69)70)21(106-43)13-100-126(53,54)55)111-45-40(123-140(95,96)97)35(119-136(83,84)85)30(117-134(77,78)79)24(108-45)16-103-129(62,63)64/h3-4,6-9,20-24,26-46H,5,10-16H2,1-2H3,(H,50,51,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64)(H,65,66,67)(H,68,69,70)(H,71,72,73)(H,74,75,76)(H,77,78,79)(H,80,81,82)(H,83,84,85)(H,86,87,88)(H,89,90,91)(H,92,93,94)(H,95,96,97)/p-16/t20-,21-,22-,23-,24-,26-,27-,28-,29-,30-,31+,32+,33+,34+,35+,36+,37+,38+,39+,40+,41+,42-,43-,44-,45-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50307393
(3-(4-Phenyl[1,2,3]triazol-1-yl)propyl 2,3,4,6-Tetr...)Show SMILES [O-]S(=O)(=O)OC[C@H]1O[C@H](OCCCn2cc(nn2)-c2ccccc2)[C@@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]3O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]4O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]4OS([O-])(=O)=O)[C@@H]3OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O |r| Show InChI InChI=1S/C35H53N3O60S13/c39-99(40,41)79-10-16-20(90-103(51,52)53)24(88-35-31(98-111(75,76)77)27(95-108(66,67)68)23(93-106(60,61)62)19(86-35)13-82-102(48,49)50)29(96-109(69,70)71)33(84-16)87-25-21(91-104(54,55)56)17(11-80-100(42,43)44)85-34(30(25)97-110(72,73)74)89-28-26(94-107(63,64)65)22(92-105(57,58)59)18(12-81-101(45,46)47)83-32(28)78-8-4-7-38-9-15(36-37-38)14-5-2-1-3-6-14/h1-3,5-6,9,16-35H,4,7-8,10-13H2,(H,39,40,41)(H,42,43,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56)(H,57,58,59)(H,60,61,62)(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)/p-13/t16-,17-,18-,19-,20-,21-,22-,23-,24+,25+,26+,27+,28+,29+,30+,31+,32+,33-,34-,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant heparanase |
J Med Chem 53: 1686-99 (2010)
Article DOI: 10.1021/jm901449m
BindingDB Entry DOI: 10.7270/Q2PG1SPH |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50041457

(4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)...)Show InChI InChI=1S/C20H22ClN3O/c1-3-24(4-2)13-14-11-16(6-8-20(14)25)23-18-9-10-22-19-12-15(21)5-7-17(18)19/h5-12,25H,3-4,13H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.26E+4 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431646
(CHEMBL330684)Show SMILES OCc1cc(NC(=O)CN2CCOCC2)cc(Nc2ccnc3cc(Cl)ccc23)c1 Show InChI InChI=1S/C22H23ClN4O3/c23-16-1-2-19-20(3-4-24-21(19)11-16)25-17-9-15(14-28)10-18(12-17)26-22(29)13-27-5-7-30-8-6-27/h1-4,9-12,28H,5-8,13-14H2,(H,24,25)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431645

(CHEMBL96248)Show SMILES Clc1ccc2c(Nc3cc(COC(=O)CN4CCCCC4)cc(NC(=O)CN4CCCCC4)c3)ccnc2c1 Show InChI InChI=1S/C30H36ClN5O3/c31-23-7-8-26-27(9-10-32-28(26)17-23)33-24-15-22(21-39-30(38)20-36-13-5-2-6-14-36)16-25(18-24)34-29(37)19-35-11-3-1-4-12-35/h7-10,15-18H,1-6,11-14,19-21H2,(H,32,33)(H,34,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431648

(CHEMBL2349233)Show InChI InChI=1S/C22H24ClN3O/c1-2-27-22-8-6-18(13-16(22)15-26-11-3-4-12-26)25-20-9-10-24-21-14-17(23)5-7-19(20)21/h5-10,13-14H,2-4,11-12,15H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.69E+5 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431650
(CHEMBL2349235)Show SMILES CCN(CC)Cc1cc(Nc2ccnc3cc(Cl)ccc23)ccc1N1CCOCC1 Show InChI InChI=1S/C24H29ClN4O/c1-3-28(4-2)17-18-15-20(6-8-24(18)29-11-13-30-14-12-29)27-22-9-10-26-23-16-19(25)5-7-21(22)23/h5-10,15-16H,3-4,11-14,17H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.39E+5 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431643
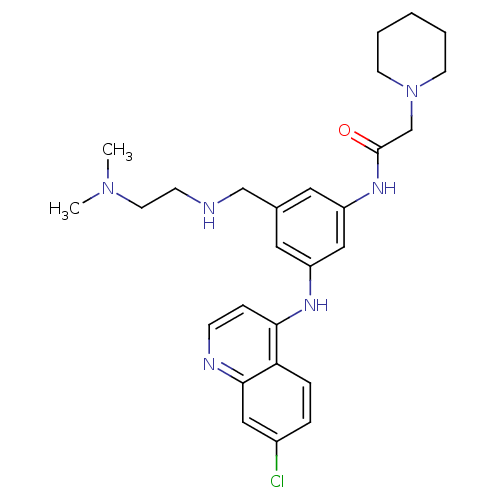
(CHEMBL319756)Show SMILES CN(C)CCNCc1cc(NC(=O)CN2CCCCC2)cc(Nc2ccnc3cc(Cl)ccc23)c1 Show InChI InChI=1S/C27H35ClN6O/c1-33(2)13-10-29-18-20-14-22(31-25-8-9-30-26-16-21(28)6-7-24(25)26)17-23(15-20)32-27(35)19-34-11-4-3-5-12-34/h6-9,14-17,29H,3-5,10-13,18-19H2,1-2H3,(H,30,31)(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.39E+5 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM61776
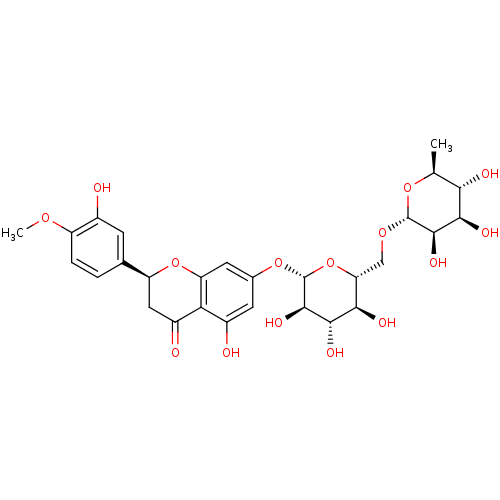
((2S)-2-(4-methoxy-3-oxidanyl-phenyl)-7-[(2S,3R,4S,...)Show SMILES COc1ccc(cc1O)[C@@H]1CC(=O)c2c(O)cc(O[C@@H]3O[C@H](CO[C@@H]4O[C@@H](C)[C@H](O)[C@@H](O)[C@H]4O)[C@@H](O)[C@H](O)[C@H]3O)cc2O1 Show InChI InChI=1S/C28H34O15/c1-10-21(32)23(34)25(36)27(40-10)39-9-19-22(33)24(35)26(37)28(43-19)41-12-6-14(30)20-15(31)8-17(42-18(20)7-12)11-3-4-16(38-2)13(29)5-11/h3-7,10,17,19,21-30,32-37H,8-9H2,1-2H3/t10-,17-,19+,21-,22+,23+,24-,25+,26+,27+,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50388686
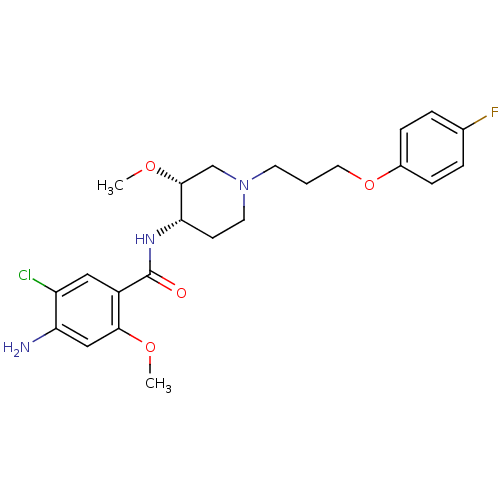
(CHEMBL74656)Show SMILES CO[C@@H]1CN(CCCOc2ccc(F)cc2)CC[C@@H]1NC(=O)c1cc(Cl)c(N)cc1OC |r| Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29)/t20-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50241582
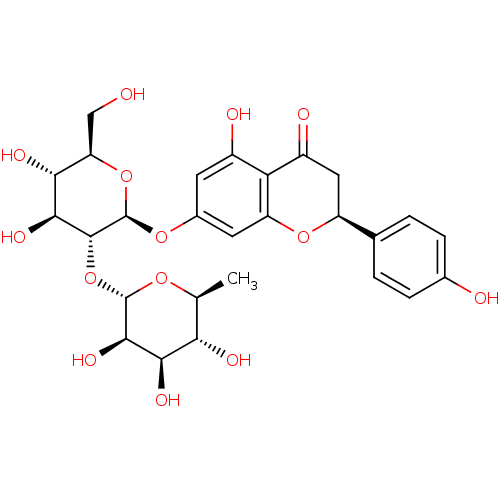
((S)-7-((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxyme...)Show SMILES C[C@@H]1O[C@@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2Oc2cc(O)c3C(=O)C[C@H](Oc3c2)c2ccc(O)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H32O14/c1-10-20(32)22(34)24(36)26(37-10)41-25-23(35)21(33)18(9-28)40-27(25)38-13-6-14(30)19-15(31)8-16(39-17(19)7-13)11-2-4-12(29)5-3-11/h2-7,10,16,18,20-30,32-36H,8-9H2,1H3/t10-,16-,18+,20-,21+,22+,23-,24+,25+,26-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM30704
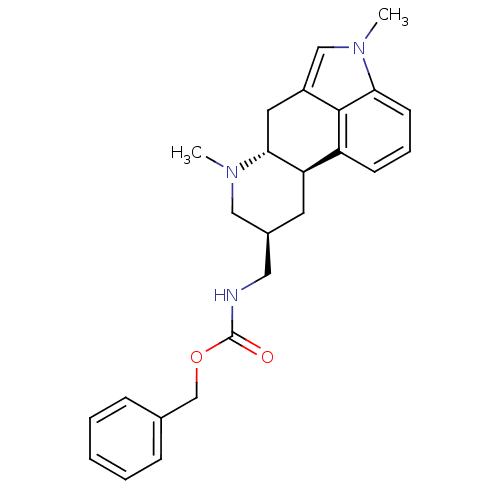
((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM25758
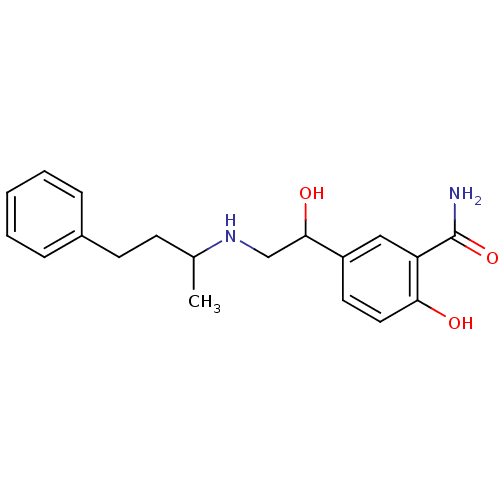
(2-hydroxy-5-{1-hydroxy-2-[(4-phenylbutan-2-yl)amin...)Show InChI InChI=1S/C19H24N2O3/c1-13(7-8-14-5-3-2-4-6-14)21-12-18(23)15-9-10-17(22)16(11-15)19(20)24/h2-6,9-11,13,18,21-23H,7-8,12H2,1H3,(H2,20,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431649

(CHEMBL2349234)Show InChI InChI=1S/C20H21BrClN3/c1-3-25(4-2)13-14-11-16(6-8-18(14)21)24-19-9-10-23-20-12-15(22)5-7-17(19)20/h5-12H,3-4,13H2,1-2H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431647
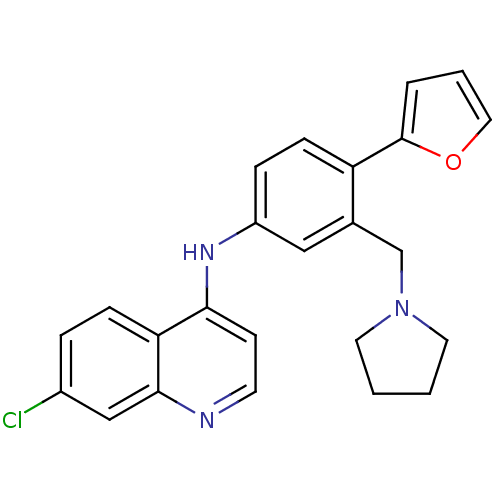
(CHEMBL2349232)Show SMILES Clc1ccc2c(Nc3ccc(-c4ccco4)c(CN4CCCC4)c3)ccnc2c1 Show InChI InChI=1S/C24H22ClN3O/c25-18-5-7-21-22(9-10-26-23(21)15-18)27-19-6-8-20(24-4-3-13-29-24)17(14-19)16-28-11-1-2-12-28/h3-10,13-15H,1-2,11-12,16H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431644
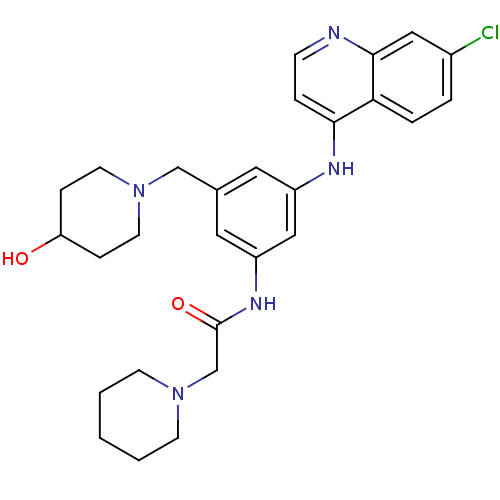
(CHEMBL94587)Show SMILES OC1CCN(Cc2cc(NC(=O)CN3CCCCC3)cc(Nc3ccnc4cc(Cl)ccc34)c2)CC1 Show InChI InChI=1S/C28H34ClN5O2/c29-21-4-5-25-26(6-9-30-27(25)16-21)31-22-14-20(18-34-12-7-24(35)8-13-34)15-23(17-22)32-28(36)19-33-10-2-1-3-11-33/h4-6,9,14-17,24,35H,1-3,7-8,10-13,18-19H2,(H,30,31)(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431641

(CHEMBL490277)Show SMILES Clc1ccc2c(Nc3cc(NC(=O)CN4CCCCC4)cc(c3)C(=O)N3CCOCC3)ccnc2c1 Show InChI InChI=1S/C27H30ClN5O3/c28-20-4-5-23-24(6-7-29-25(23)16-20)30-21-14-19(27(35)33-10-12-36-13-11-33)15-22(17-21)31-26(34)18-32-8-2-1-3-9-32/h4-7,14-17H,1-3,8-13,18H2,(H,29,30)(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431642
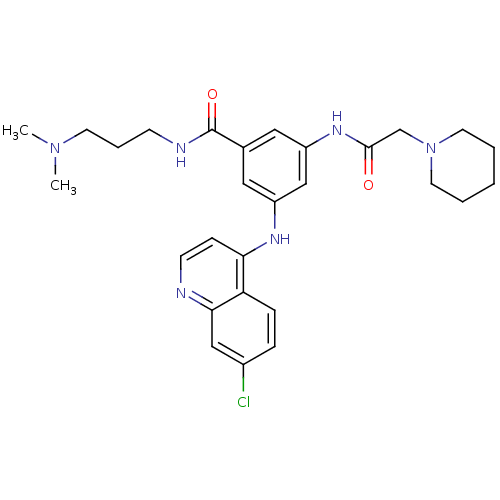
(CHEMBL475535)Show SMILES CN(C)CCCNC(=O)c1cc(NC(=O)CN2CCCCC2)cc(Nc2ccnc3cc(Cl)ccc23)c1 Show InChI InChI=1S/C28H35ClN6O2/c1-34(2)12-6-10-31-28(37)20-15-22(32-25-9-11-30-26-17-21(29)7-8-24(25)26)18-23(16-20)33-27(36)19-35-13-4-3-5-14-35/h7-9,11,15-18H,3-6,10,12-14,19H2,1-2H3,(H,30,32)(H,31,37)(H,33,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) by surface plasmon resonance assa... |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

































