 Found 121 hits of ic50 for UniProtKB: P08424
Found 121 hits of ic50 for UniProtKB: P08424 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Rattus norvegicus) | BDBM50014100

(2-(1-{[1-{2-Hydroxy-1-isobutyl-5-methyl-4-[2-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C50H75N9O8/c1-11-33(6)43(47(64)53-29-35-20-15-16-22-52-35)57-44(61)37(32(4)5)27-42(60)38(24-31(2)3)55-46(63)41(26-36-28-51-30-54-36)58(10)48(65)39(25-34-18-13-12-14-19-34)56-45(62)40-21-17-23-59(40)49(66)67-50(7,8)9/h12-16,18-20,22,28,30-33,37-43,60H,11,17,21,23-27,29H2,1-10H3,(H,51,54)(H,53,64)(H,55,63)(H,56,62)(H,57,61)/t33-,37+,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Tested invitro for inhibitory activity against plasma renin in rat models |
J Med Chem 33: 2276-83 (1990)
BindingDB Entry DOI: 10.7270/Q28C9V7S |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50014102
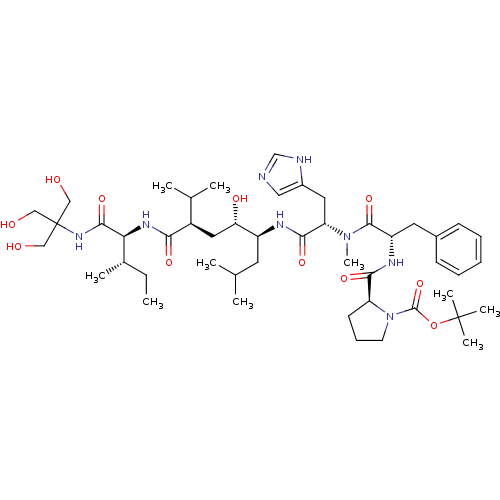
(Boc-Pro-Phe-N(Me) His-Leu[CHOHCH2]Val-Ile-THAM Ami...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(C)C)C(=O)NC(CO)(CO)CO Show InChI InChI=1S/C48H78N8O11/c1-11-31(6)40(44(64)54-48(25-57,26-58)27-59)53-41(61)34(30(4)5)23-39(60)35(20-29(2)3)51-43(63)38(22-33-24-49-28-50-33)55(10)45(65)36(21-32-16-13-12-14-17-32)52-42(62)37-18-15-19-56(37)46(66)67-47(7,8)9/h12-14,16-17,24,28-31,34-40,57-60H,11,15,18-23,25-27H2,1-10H3,(H,49,50)(H,51,63)(H,52,62)(H,53,61)(H,54,64)/t31-,34+,35-,36-,37-,38-,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Tested invitro for inhibitory activity against plasma renin in rat models |
J Med Chem 33: 2276-83 (1990)
BindingDB Entry DOI: 10.7270/Q28C9V7S |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50006844
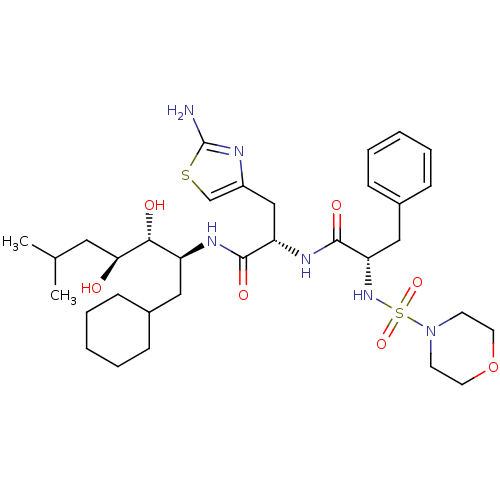
((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1csc(N)n1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C33H52N6O7S2/c1-22(2)17-29(40)30(41)26(18-23-9-5-3-6-10-23)36-31(42)27(20-25-21-47-33(34)35-25)37-32(43)28(19-24-11-7-4-8-12-24)38-48(44,45)39-13-15-46-16-14-39/h4,7-8,11-12,21-23,26-30,38,40-41H,3,5-6,9-10,13-20H2,1-2H3,(H2,34,35)(H,36,42)(H,37,43)/t26-,27-,28-,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against renin was determined |
Bioorg Med Chem Lett 3: 813-818 (1993)
Article DOI: 10.1016/S0960-894X(00)80672-1
BindingDB Entry DOI: 10.7270/Q261107B |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50014106
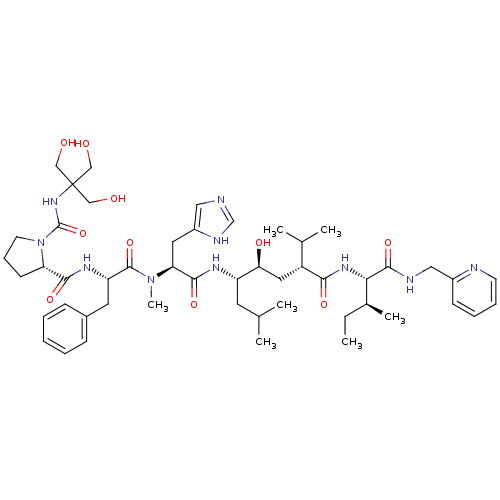
(CHEMBL429882 | N-[[[2-Hydroxy-1,1-bis(hydroxymethy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)NC(CO)(CO)CO)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C50H76N10O10/c1-8-33(6)43(47(68)53-26-35-17-12-13-19-52-35)57-44(65)37(32(4)5)24-42(64)38(21-31(2)3)55-46(67)41(23-36-25-51-30-54-36)59(7)48(69)39(22-34-15-10-9-11-16-34)56-45(66)40-18-14-20-60(40)49(70)58-50(27-61,28-62)29-63/h9-13,15-17,19,25,30-33,37-43,61-64H,8,14,18,20-24,26-29H2,1-7H3,(H,51,54)(H,53,68)(H,55,67)(H,56,66)(H,57,65)(H,58,70)/t33-,37+,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Tested invitro for inhibitory activity against plasma renin in rat models |
J Med Chem 33: 2276-83 (1990)
BindingDB Entry DOI: 10.7270/Q28C9V7S |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50014105
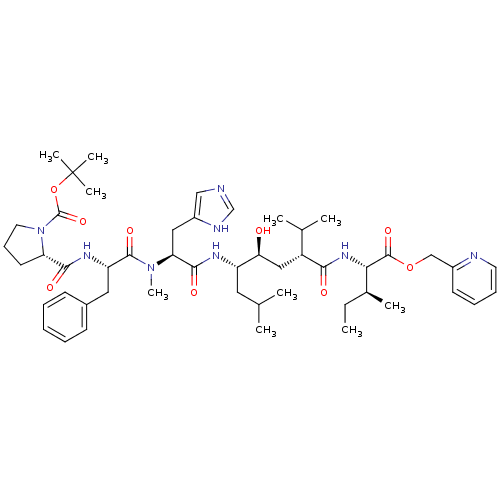
(2-(1-{[1-{2-Hydroxy-1-isobutyl-5-methyl-4-[2-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(C)C)C(=O)OCc1ccccn1 Show InChI InChI=1S/C50H74N8O9/c1-11-33(6)43(48(64)66-29-35-20-15-16-22-52-35)56-44(60)37(32(4)5)27-42(59)38(24-31(2)3)54-46(62)41(26-36-28-51-30-53-36)57(10)47(63)39(25-34-18-13-12-14-19-34)55-45(61)40-21-17-23-58(40)49(65)67-50(7,8)9/h12-16,18-20,22,28,30-33,37-43,59H,11,17,21,23-27,29H2,1-10H3,(H,51,53)(H,54,62)(H,55,61)(H,56,60)/t33-,37+,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Tested invitro for inhibitory activity against plasma renin in rat models |
J Med Chem 33: 2276-83 (1990)
BindingDB Entry DOI: 10.7270/Q28C9V7S |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50014099
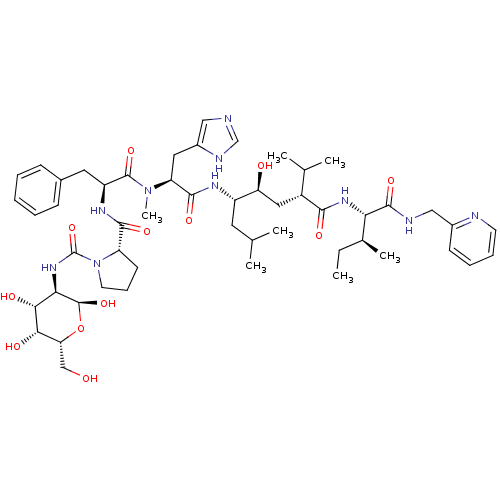
(CHEMBL414795 | N-[(D-Glucosylamino)carbonyl]-Pro-P...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C52H78N10O12/c1-8-31(6)42(49(70)55-26-33-17-12-13-19-54-33)59-46(67)35(30(4)5)24-40(64)36(21-29(2)3)57-48(69)39(23-34-25-53-28-56-34)61(7)50(71)37(22-32-15-10-9-11-16-32)58-47(68)38-18-14-20-62(38)52(73)60-43-45(66)44(65)41(27-63)74-51(43)72/h9-13,15-17,19,25,28-31,35-45,51,63-66,72H,8,14,18,20-24,26-27H2,1-7H3,(H,53,56)(H,55,70)(H,57,69)(H,58,68)(H,59,67)(H,60,73)/t31-,35+,36-,37-,38-,39-,40-,41+,42-,43+,44-,45+,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Tested invitro for inhibitory activity against plasma renin in rat models |
J Med Chem 33: 2276-83 (1990)
BindingDB Entry DOI: 10.7270/Q28C9V7S |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50014104
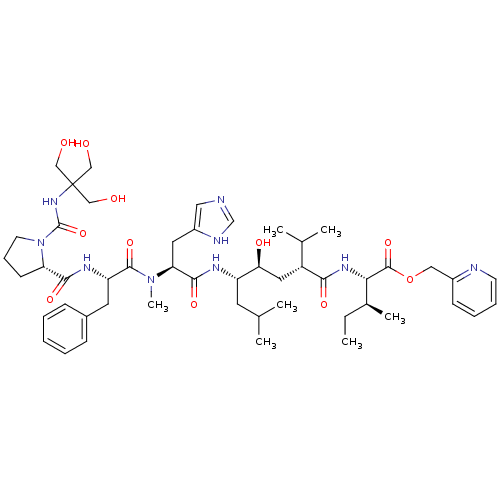
(CHEMBL410067 | N-[[[2-Hydroxy-1,1-bis(hydroxymethy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)NC(CO)(CO)CO)C(C)C)C(=O)OCc1ccccn1 Show InChI InChI=1S/C50H75N9O11/c1-8-33(6)43(48(68)70-26-35-17-12-13-19-52-35)56-44(64)37(32(4)5)24-42(63)38(21-31(2)3)54-46(66)41(23-36-25-51-30-53-36)58(7)47(67)39(22-34-15-10-9-11-16-34)55-45(65)40-18-14-20-59(40)49(69)57-50(27-60,28-61)29-62/h9-13,15-17,19,25,30-33,37-43,60-63H,8,14,18,20-24,26-29H2,1-7H3,(H,51,53)(H,54,66)(H,55,65)(H,56,64)(H,57,69)/t33-,37+,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Tested invitro for inhibitory activity against plasma renin in rat models |
J Med Chem 33: 2276-83 (1990)
BindingDB Entry DOI: 10.7270/Q28C9V7S |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50014103
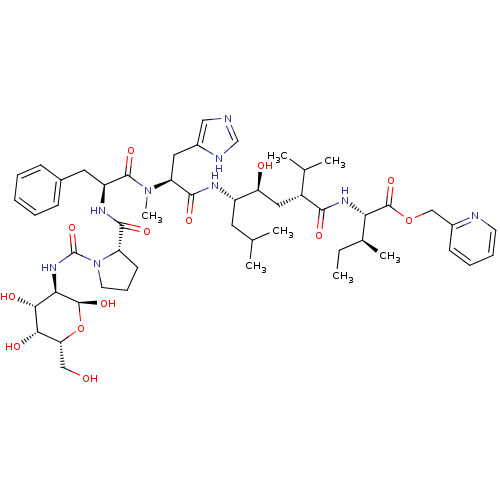
(CHEMBL263257 | N-[(D-Glucosylamino)carbonyl]-Pro-P...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O)C(C)C)C(=O)OCc1ccccn1 Show InChI InChI=1S/C52H77N9O13/c1-8-31(6)42(50(70)73-27-33-17-12-13-19-54-33)58-46(66)35(30(4)5)24-40(63)36(21-29(2)3)56-48(68)39(23-34-25-53-28-55-34)60(7)49(69)37(22-32-15-10-9-11-16-32)57-47(67)38-18-14-20-61(38)52(72)59-43-45(65)44(64)41(26-62)74-51(43)71/h9-13,15-17,19,25,28-31,35-45,51,62-65,71H,8,14,18,20-24,26-27H2,1-7H3,(H,53,55)(H,56,68)(H,57,67)(H,58,66)(H,59,72)/t31-,35+,36-,37-,38-,39-,40-,41+,42-,43+,44-,45+,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Tested invitro for inhibitory activity against plasma renin in rat models |
J Med Chem 33: 2276-83 (1990)
BindingDB Entry DOI: 10.7270/Q28C9V7S |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50014101

(CHEMBL265833 | N-[4,5-Dihydro-4,4-bis(hydroxymethy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C1=NC(CO)(CO)CO1)C(C)C)C(=O)OCc1ccccn1 |t:50| Show InChI InChI=1S/C50H73N9O10/c1-8-33(6)43(48(67)68-26-35-17-12-13-19-52-35)56-44(63)37(32(4)5)24-42(62)38(21-31(2)3)54-46(65)41(23-36-25-51-30-53-36)58(7)47(66)39(22-34-15-10-9-11-16-34)55-45(64)40-18-14-20-59(40)49-57-50(27-60,28-61)29-69-49/h9-13,15-17,19,25,30-33,37-43,60-62H,8,14,18,20-24,26-29H2,1-7H3,(H,51,53)(H,54,65)(H,55,64)(H,56,63)/t33-,37+,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
Tested invitro for inhibitory activity against plasma renin in rat models |
J Med Chem 33: 2276-83 (1990)
BindingDB Entry DOI: 10.7270/Q28C9V7S |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50403370
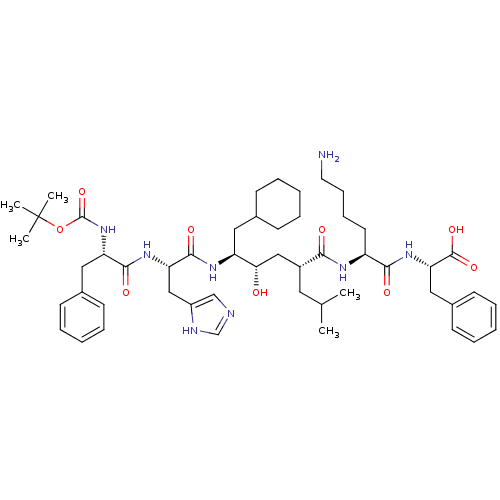
(CHEMBL2052021 | CP-71362)Show SMILES CC(C)C[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C51H76N8O9/c1-33(2)25-37(45(61)55-39(23-15-16-24-52)46(62)58-43(49(65)66)28-36-21-13-8-14-22-36)29-44(60)40(26-34-17-9-6-10-18-34)56-48(64)42(30-38-31-53-32-54-38)57-47(63)41(27-35-19-11-7-12-20-35)59-50(67)68-51(3,4)5/h7-8,11-14,19-22,31-34,37,39-44,60H,6,9-10,15-18,23-30,52H2,1-5H3,(H,53,54)(H,55,61)(H,56,64)(H,57,63)(H,58,62)(H,59,67)(H,65,66)/t37-,39+,40+,41+,42+,43+,44+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against Rat plasma renin |
Bioorg Med Chem Lett 4: 589-592 (1994)
Article DOI: 10.1016/S0960-894X(01)80160-8
BindingDB Entry DOI: 10.7270/Q2KD1XVS |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022846
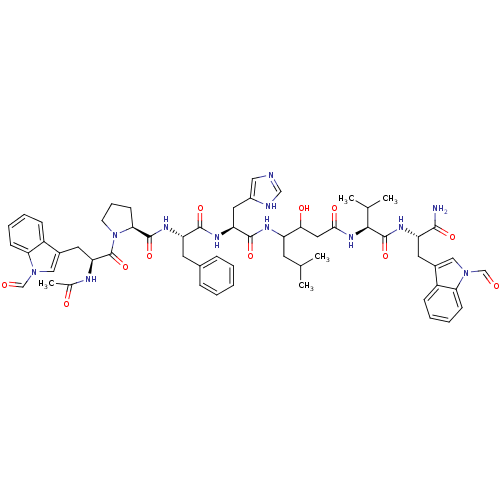
(Ac-Ftr-Pro-Phe-His-Sta-Val-Ftr-NH2 | CHEMBL275711)Show SMILES CC(C)CC(NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(C)=O)C(O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cn(C=O)c2ccccc12)C(N)=O Show InChI InChI=1S/C59H72N12O11/c1-34(2)22-43(51(75)27-52(76)68-53(35(3)4)58(81)65-44(54(60)77)24-38-29-69(32-72)48-18-11-9-16-41(38)48)64-56(79)46(26-40-28-61-31-62-40)66-55(78)45(23-37-14-7-6-8-15-37)67-57(80)50-20-13-21-71(50)59(82)47(63-36(5)74)25-39-30-70(33-73)49-19-12-10-17-42(39)49/h6-12,14-19,28-35,43-47,50-51,53,75H,13,20-27H2,1-5H3,(H2,60,77)(H,61,62)(H,63,74)(H,64,79)(H,65,81)(H,66,78)(H,67,80)(H,68,76)/t43?,44-,45-,46-,47-,50-,51?,53-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards porcine kidney renin |
J Med Chem 31: 18-30 (1988)
BindingDB Entry DOI: 10.7270/Q2C53MDQ |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024163

(4-[2-(4-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C44H64N6O10/c1-25(2)18-33(36(51)21-38(53)46-28(7)41(55)47-34(19-26(3)4)37(52)22-39(54)59-8)48-43(57)40(27(5)6)50-42(56)35(20-30-23-45-32-17-13-12-16-31(30)32)49-44(58)60-24-29-14-10-9-11-15-29/h9-17,23,25-28,33-37,40,45,51-52H,18-22,24H2,1-8H3,(H,46,53)(H,47,55)(H,48,57)(H,49,58)(H,50,56) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against man (human) plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024163

(4-[2-(4-{2-[2-Benzyloxycarbonylamino-3-(1H-indol-3...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OCc1ccccc1)C(C)C |r| Show InChI InChI=1S/C44H64N6O10/c1-25(2)18-33(36(51)21-38(53)46-28(7)41(55)47-34(19-26(3)4)37(52)22-39(54)59-8)48-43(57)40(27(5)6)50-42(56)35(20-30-23-45-32-17-13-12-16-31(30)32)49-44(58)60-24-29-14-10-9-11-15-29/h9-17,23,25-28,33-37,40,45,51-52H,18-22,24H2,1-8H3,(H,46,53)(H,47,55)(H,48,57)(H,49,58)(H,50,56) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of dog plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022002
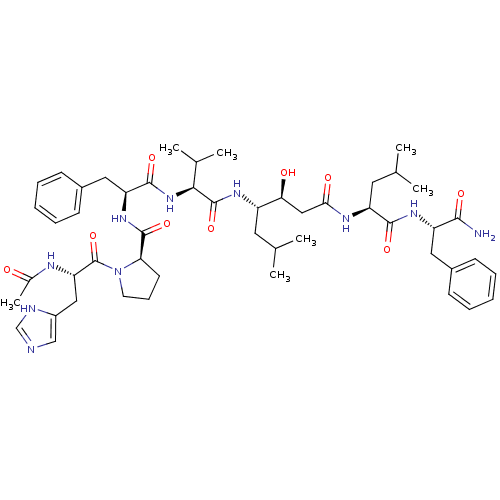
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H72N10O9/c1-29(2)21-36(42(62)26-43(63)55-38(22-30(3)4)46(65)57-37(45(51)64)23-33-15-10-8-11-16-33)56-49(68)44(31(5)6)59-47(66)39(24-34-17-12-9-13-18-34)58-48(67)41-19-14-20-60(41)50(69)40(54-32(7)61)25-35-27-52-28-53-35/h8-13,15-18,27-31,36-42,44,62H,14,19-26H2,1-7H3,(H2,51,64)(H,52,53)(H,54,61)(H,55,63)(H,56,68)(H,57,65)(H,58,67)(H,59,66)/t36-,37-,38-,39-,40-,41+,42-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50421761
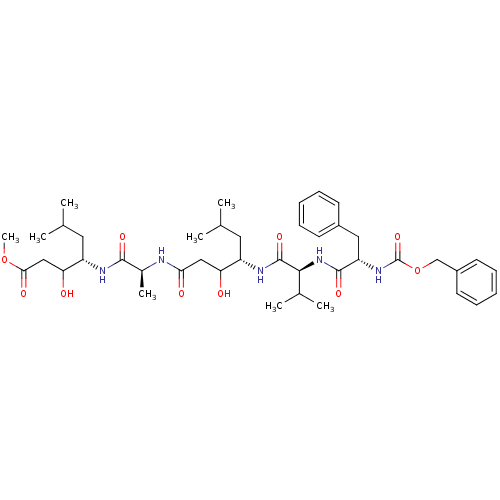
(CHEMBL2311113)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C42H63N5O10/c1-25(2)19-31(34(48)22-36(50)43-28(7)39(52)44-32(20-26(3)4)35(49)23-37(51)56-8)45-41(54)38(27(5)6)47-40(53)33(21-29-15-11-9-12-16-29)46-42(55)57-24-30-17-13-10-14-18-30/h9-18,25-28,31-35,38,48-49H,19-24H2,1-8H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53)/t28-,31-,32-,33-,34?,35?,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against hog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022837

(CHEMBL437836 | Iva--His-Pro-Phe-His-Sta-Leu-Phe-NH...)Show SMILES CCC(C)(N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)NC(CC(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H77N13O9/c1-7-54(6,56)53(76)66-43(26-37-29-58-31-60-37)52(75)67-20-14-19-44(67)51(74)65-41(24-35-17-12-9-13-18-35)49(72)64-42(25-36-28-57-30-59-36)50(73)62-38(21-32(2)3)45(68)27-46(69)61-40(22-33(4)5)48(71)63-39(47(55)70)23-34-15-10-8-11-16-34/h8-13,15-18,28-33,38-45,68H,7,14,19-27,56H2,1-6H3,(H2,55,70)(H,57,59)(H,58,60)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)(H,66,76)/t38?,39-,40-,41-,42-,43-,44-,45?,54?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards porcine kidney renin |
J Med Chem 31: 18-30 (1988)
BindingDB Entry DOI: 10.7270/Q2C53MDQ |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022007
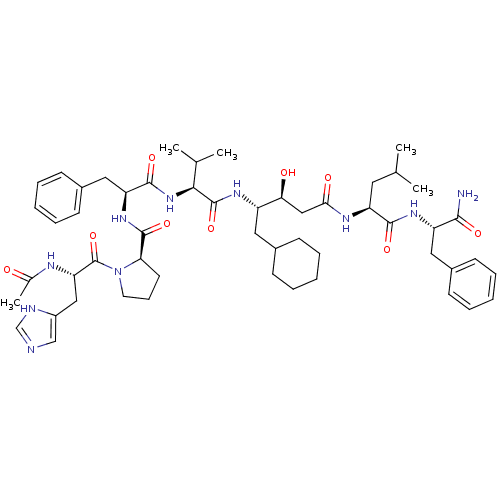
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H76N10O9/c1-32(2)24-41(49(68)60-40(48(54)67)26-36-18-11-7-12-19-36)58-46(66)29-45(65)39(25-35-16-9-6-10-17-35)59-52(71)47(33(3)4)62-50(69)42(27-37-20-13-8-14-21-37)61-51(70)44-22-15-23-63(44)53(72)43(57-34(5)64)28-38-30-55-31-56-38/h7-8,11-14,18-21,30-33,35,39-45,47,65H,6,9-10,15-17,22-29H2,1-5H3,(H2,54,67)(H,55,56)(H,57,64)(H,58,66)(H,59,71)(H,60,68)(H,61,70)(H,62,69)/t39-,40-,41-,42-,43-,44+,45-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021993

(1-[2-Acetylamino-3-(4-methoxy-phenyl)-propionyl]-p...)Show SMILES COc1ccc(C[C@H](NC(C)=O)C(=O)N2CCC[C@@H]2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)cc1 Show InChI InChI=1S/C54H76N8O10/c1-32(2)26-40(46(64)31-47(65)57-42(27-33(3)4)50(67)59-41(49(55)66)28-36-16-11-9-12-17-36)58-53(70)48(34(5)6)61-51(68)43(29-37-18-13-10-14-19-37)60-52(69)45-20-15-25-62(45)54(71)44(56-35(7)63)30-38-21-23-39(72-8)24-22-38/h9-14,16-19,21-24,32-34,40-46,48,64H,15,20,25-31H2,1-8H3,(H2,55,66)(H,56,63)(H,57,65)(H,58,70)(H,59,67)(H,60,69)(H,61,68)/t40-,41-,42-,43-,44-,45+,46-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024167
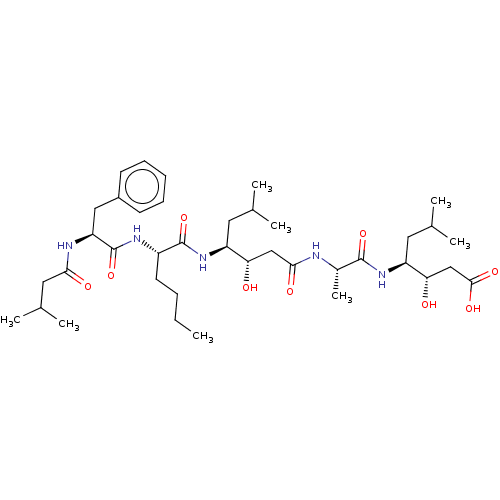
(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against hog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50421761

(CHEMBL2311113)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C42H63N5O10/c1-25(2)19-31(34(48)22-36(50)43-28(7)39(52)44-32(20-26(3)4)35(49)23-37(51)56-8)45-41(54)38(27(5)6)47-40(53)33(21-29-15-11-9-12-16-29)46-42(55)57-24-30-17-13-10-14-18-30/h9-18,25-28,31-35,38,48-49H,19-24H2,1-8H3,(H,43,50)(H,44,52)(H,45,54)(H,46,55)(H,47,53)/t28-,31-,32-,33-,34?,35?,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50144378
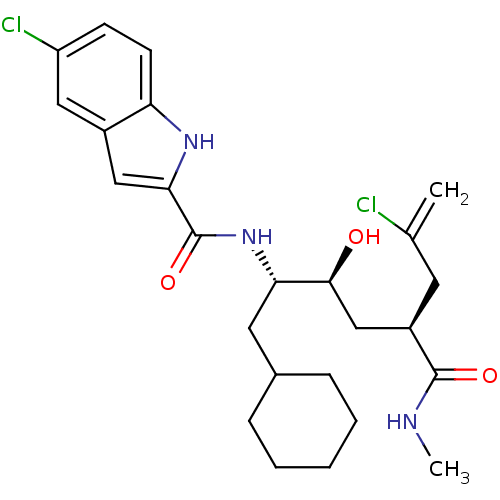
(5-Chloro-1H-indole-2-carboxylic acid ((1S,2S,4S)-6...)Show SMILES CNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1cc2cc(Cl)ccc2[nH]1)CC(Cl)=C Show InChI InChI=1S/C25H33Cl2N3O3/c1-15(26)10-18(24(32)28-2)14-23(31)21(11-16-6-4-3-5-7-16)30-25(33)22-13-17-12-19(27)8-9-20(17)29-22/h8-9,12-13,16,18,21,23,29,31H,1,3-7,10-11,14H2,2H3,(H,28,32)(H,30,33)/t18-,21+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against renin was determined |
Bioorg Med Chem Lett 14: 2163-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.020
BindingDB Entry DOI: 10.7270/Q2CV4H5M |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022021
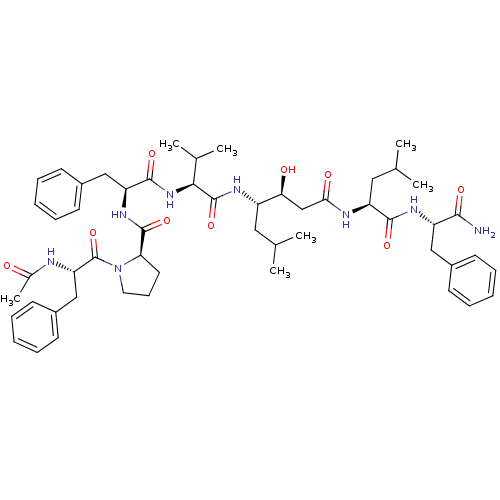
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H74N8O9/c1-32(2)26-39(45(63)31-46(64)56-41(27-33(3)4)49(66)58-40(48(54)65)28-36-18-11-8-12-19-36)57-52(69)47(34(5)6)60-50(67)42(29-37-20-13-9-14-21-37)59-51(68)44-24-17-25-61(44)53(70)43(55-35(7)62)30-38-22-15-10-16-23-38/h8-16,18-23,32-34,39-45,47,63H,17,24-31H2,1-7H3,(H2,54,65)(H,55,62)(H,56,64)(H,57,69)(H,58,66)(H,59,68)(H,60,67)/t39-,40-,41-,42-,43-,44+,45-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024165
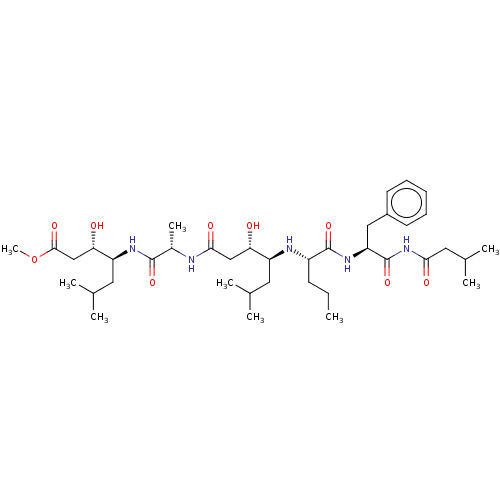
(4-[2-(4-{1-[1-Benzyl-2-(3-methyl-butyrylamino)-2-o...)Show SMILES CCC[C@H](N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)OC)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC(=O)CC(C)C |r| Show InChI InChI=1S/C39H65N5O9/c1-10-14-28(38(51)43-31(20-27-15-12-11-13-16-27)39(52)44-34(47)19-25(6)7)41-29(17-23(2)3)32(45)21-35(48)40-26(8)37(50)42-30(18-24(4)5)33(46)22-36(49)53-9/h11-13,15-16,23-26,28-33,41,45-46H,10,14,17-22H2,1-9H3,(H,40,48)(H,42,50)(H,43,51)(H,44,47,52) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against hog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021989
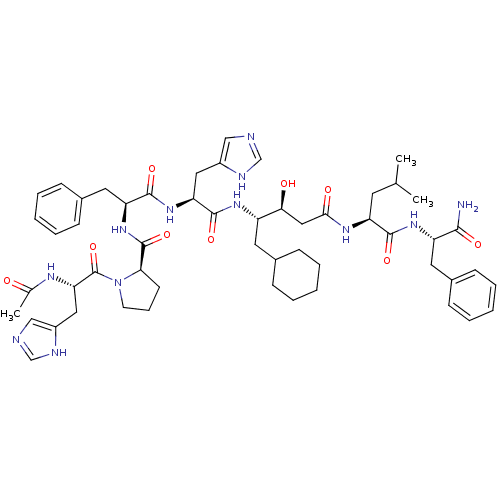
(Ac-His-Pro-Phe-His-ACHPA-Leu-Phe-NH2 | CHEMBL38686...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H74N12O9/c1-33(2)22-42(50(71)63-41(49(55)70)24-36-16-9-5-10-17-36)61-48(69)28-47(68)40(23-35-14-7-4-8-15-35)62-52(73)44(26-38-29-56-31-58-38)64-51(72)43(25-37-18-11-6-12-19-37)65-53(74)46-20-13-21-66(46)54(75)45(60-34(3)67)27-39-30-57-32-59-39/h5-6,9-12,16-19,29-33,35,40-47,68H,4,7-8,13-15,20-28H2,1-3H3,(H2,55,70)(H,56,58)(H,57,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t40-,41-,42-,43-,44-,45-,46+,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022015

(1-(2-Acetylamino-3-naphthalen-1-yl-propionyl)-pyrr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cccc2ccccc12)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H76N8O9/c1-34(2)28-43(49(67)33-50(68)60-45(29-35(3)4)53(70)62-44(52(58)69)30-38-18-10-8-11-19-38)61-56(73)51(36(5)6)64-54(71)46(31-39-20-12-9-13-21-39)63-55(72)48-26-17-27-65(48)57(74)47(59-37(7)66)32-41-24-16-23-40-22-14-15-25-42(40)41/h8-16,18-25,34-36,43-49,51,67H,17,26-33H2,1-7H3,(H2,58,69)(H,59,66)(H,60,68)(H,61,73)(H,62,70)(H,63,72)(H,64,71)/t43-,44-,45-,46-,47-,48+,49-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024174
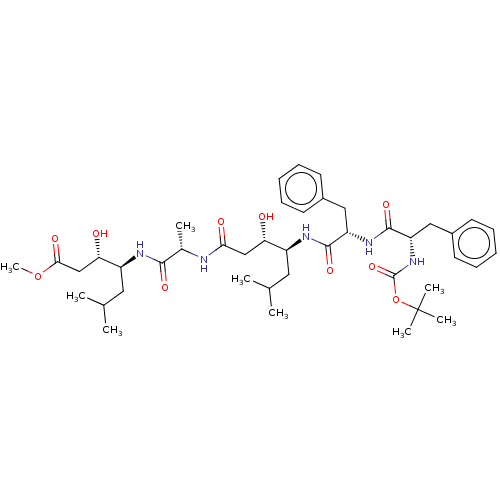
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H65N5O10/c1-26(2)20-31(35(49)24-37(51)44-28(5)39(53)45-32(21-27(3)4)36(50)25-38(52)57-9)46-40(54)33(22-29-16-12-10-13-17-29)47-41(55)34(23-30-18-14-11-15-19-30)48-42(56)58-43(6,7)8/h10-19,26-28,31-36,49-50H,20-25H2,1-9H3,(H,44,51)(H,45,53)(H,46,54)(H,47,55)(H,48,56) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against hog plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50367761

(CHEMBL1790252)Show SMILES CC[C@@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H79N13O9/c1-3-35(2)50(56(78)65-43(51(58)73)26-37-17-9-5-10-18-37)69-49(72)30-48(71)42(25-36-15-7-4-8-16-36)64-54(76)45(28-39-31-59-33-62-39)66-53(75)44(27-38-19-11-6-12-20-38)67-55(77)47-22-14-24-70(47)57(79)46(29-40-32-60-34-63-40)68-52(74)41-21-13-23-61-41/h5-6,9-12,17-20,31-36,41-48,50,61,71H,3-4,7-8,13-16,21-30H2,1-2H3,(H2,58,73)(H,59,62)(H,60,63)(H,64,76)(H,65,78)(H,66,75)(H,67,77)(H,68,74)(H,69,72)/t35-,41-,42+,43+,44+,45+,46+,47-,48+,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corp
Curated by ChEMBL
| Assay Description
Inhibition of rat renin |
J Med Chem 53: 7490-520 (2010)
Article DOI: 10.1021/jm901885s
BindingDB Entry DOI: 10.7270/Q2S75GKG |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in rat plasma |
Bioorg Med Chem 26: 3261-3286 (2018)
Article DOI: 10.1016/j.bmc.2018.04.051
BindingDB Entry DOI: 10.7270/Q2VH5RCG |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022835
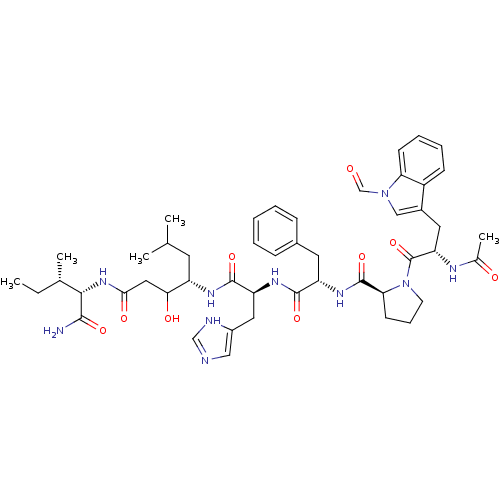
(Ac-Ftr-Pro-Phe-His-Sta-Ile-NH2 | CHEMBL419531)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(C)=O)C(N)=O Show InChI InChI=1S/C48H64N10O9/c1-6-29(4)43(44(49)63)56-42(62)23-41(61)35(19-28(2)3)53-46(65)37(22-33-24-50-26-51-33)54-45(64)36(20-31-13-8-7-9-14-31)55-47(66)40-17-12-18-58(40)48(67)38(52-30(5)60)21-32-25-57(27-59)39-16-11-10-15-34(32)39/h7-11,13-16,24-29,35-38,40-41,43,61H,6,12,17-23H2,1-5H3,(H2,49,63)(H,50,51)(H,52,60)(H,53,65)(H,54,64)(H,55,66)(H,56,62)/t29-,35-,36-,37-,38-,40-,41?,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards porcine kidney renin |
J Med Chem 31: 18-30 (1988)
BindingDB Entry DOI: 10.7270/Q2C53MDQ |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50025903
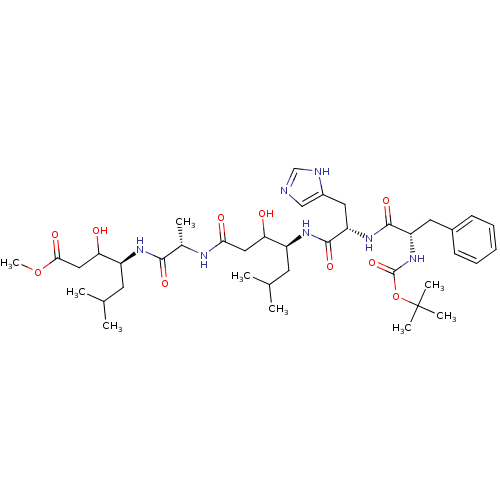
(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C40H63N7O10/c1-23(2)15-28(32(48)19-34(50)43-25(5)36(52)44-29(16-24(3)4)33(49)20-35(51)56-9)45-38(54)31(18-27-21-41-22-42-27)46-37(53)30(17-26-13-11-10-12-14-26)47-39(55)57-40(6,7)8/h10-14,21-25,28-33,48-49H,15-20H2,1-9H3,(H,41,42)(H,43,50)(H,44,52)(H,45,54)(H,46,53)(H,47,55)/t25-,28-,29-,30-,31-,32?,33?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of hog man plasma renin activity |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021994
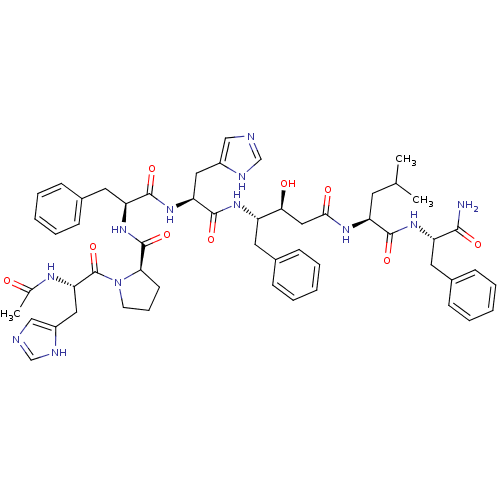
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H68N12O9/c1-33(2)22-42(50(71)63-41(49(55)70)24-36-16-9-5-10-17-36)61-48(69)28-47(68)40(23-35-14-7-4-8-15-35)62-52(73)44(26-38-29-56-31-58-38)64-51(72)43(25-37-18-11-6-12-19-37)65-53(74)46-20-13-21-66(46)54(75)45(60-34(3)67)27-39-30-57-32-59-39/h4-12,14-19,29-33,40-47,68H,13,20-28H2,1-3H3,(H2,55,70)(H,56,58)(H,57,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t40-,41-,42-,43-,44-,45-,46+,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022006

(Ac-His-Pro-Phe-His-Statine-Leu-CHA-NH2 | CHEMBL217...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C1CCCCC1)C(N)=O Show InChI InChI=1S/C50H74N12O9/c1-29(2)19-36(42(64)24-43(65)57-37(20-30(3)4)48(69)61-44(45(51)66)33-15-10-7-11-16-33)58-47(68)39(22-34-25-52-27-54-34)59-46(67)38(21-32-13-8-6-9-14-32)60-49(70)41-17-12-18-62(41)50(71)40(56-31(5)63)23-35-26-53-28-55-35/h6,8-9,13-14,25-30,33,36-42,44,64H,7,10-12,15-24H2,1-5H3,(H2,51,66)(H,52,54)(H,53,55)(H,56,63)(H,57,65)(H,58,68)(H,59,67)(H,60,70)(H,61,69)/t36-,37-,38-,39-,40-,41+,42-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024174

(4-(2-{4-[2-(2-tert-Butoxycarbonylamino-3-phenyl-pr...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C43H65N5O10/c1-26(2)20-31(35(49)24-37(51)44-28(5)39(53)45-32(21-27(3)4)36(50)25-38(52)57-9)46-40(54)33(22-29-16-12-10-13-17-29)47-41(55)34(23-30-18-14-11-15-19-30)48-42(56)58-43(6,7)8/h10-19,26-28,31-36,49-50H,20-25H2,1-9H3,(H,44,51)(H,45,53)(H,46,54)(H,47,55)(H,48,56) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50006844

((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...)Show SMILES CC(C)C[C@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1csc(N)n1)NC(=O)[C@H](Cc1ccccc1)NS(=O)(=O)N1CCOCC1 Show InChI InChI=1S/C33H52N6O7S2/c1-22(2)17-29(40)30(41)26(18-23-9-5-3-6-10-23)36-31(42)27(20-25-21-47-33(34)35-25)37-32(43)28(19-24-11-7-4-8-12-24)38-48(44,45)39-13-15-46-16-14-39/h4,7-8,11-12,21-23,26-30,38,40-41H,3,5-6,9-10,13-20H2,1-2H3,(H2,34,35)(H,36,42)(H,37,43)/t26-,27-,28-,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co.
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of plasma renin activity in rat |
J Med Chem 35: 2562-72 (1992)
BindingDB Entry DOI: 10.7270/Q2ZK5H8V |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022005

(Ac-His-Pro-Phe-His-Statine-Leu-Phe-NH2 | CHEMBL266...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H70N12O9/c1-30(2)19-37(44(65)25-45(66)58-39(20-31(3)4)47(68)60-38(46(52)67)21-33-13-8-6-9-14-33)59-49(70)41(23-35-26-53-28-55-35)61-48(69)40(22-34-15-10-7-11-16-34)62-50(71)43-17-12-18-63(43)51(72)42(57-32(5)64)24-36-27-54-29-56-36/h6-11,13-16,26-31,37-44,65H,12,17-25H2,1-5H3,(H2,52,67)(H,53,55)(H,54,56)(H,57,64)(H,58,66)(H,59,70)(H,60,68)(H,61,69)(H,62,71)/t37-,38-,39-,40-,41-,42-,43+,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022001
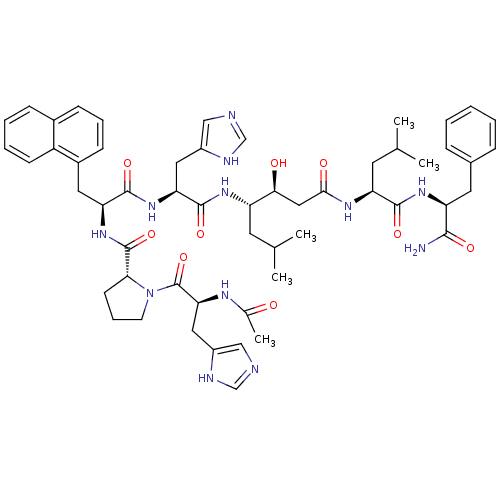
(Ac-His-Pro-Napa-His-Statine-Leu-Phe-NH2 | CHEMBL27...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C55H72N12O9/c1-32(2)21-41(48(69)27-49(70)62-43(22-33(3)4)51(72)64-42(50(56)71)23-35-13-7-6-8-14-35)63-53(74)45(25-38-28-57-30-59-38)65-52(73)44(24-37-17-11-16-36-15-9-10-18-40(36)37)66-54(75)47-19-12-20-67(47)55(76)46(61-34(5)68)26-39-29-58-31-60-39/h6-11,13-18,28-33,41-48,69H,12,19-27H2,1-5H3,(H2,56,71)(H,57,59)(H,58,60)(H,61,68)(H,62,70)(H,63,74)(H,64,72)(H,65,73)(H,66,75)/t41-,42-,43-,44-,45-,46-,47+,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022017
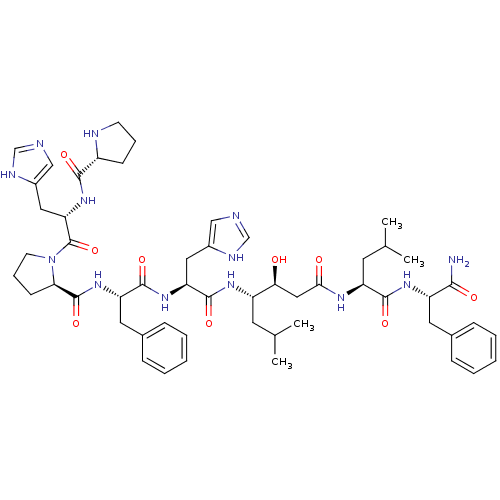
(CHEMBL414537 | Pro-His-Pro-Phe-His-Statine-Leu-Phe...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H75N13O9/c1-32(2)21-39(46(68)27-47(69)61-41(22-33(3)4)50(72)63-40(48(55)70)23-34-13-7-5-8-14-34)62-52(74)43(25-36-28-56-30-59-36)64-51(73)42(24-35-15-9-6-10-16-35)65-53(75)45-18-12-20-67(45)54(76)44(26-37-29-57-31-60-37)66-49(71)38-17-11-19-58-38/h5-10,13-16,28-33,38-46,58,68H,11-12,17-27H2,1-4H3,(H2,55,70)(H,56,59)(H,57,60)(H,61,69)(H,62,74)(H,63,72)(H,64,73)(H,65,75)(H,66,71)/t38-,39+,40+,41+,42+,43+,44+,45-,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022008

(Ac-His-Pro-Trp-His-Statine-Leu-Phe-NH2 | CHEMBL384...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H71N13O9/c1-30(2)18-39(46(68)24-47(69)61-41(19-31(3)4)49(71)63-40(48(54)70)20-33-12-7-6-8-13-33)62-51(73)43(22-35-26-55-28-58-35)64-50(72)42(21-34-25-57-38-15-10-9-14-37(34)38)65-52(74)45-16-11-17-66(45)53(75)44(60-32(5)67)23-36-27-56-29-59-36/h6-10,12-15,25-31,39-46,57,68H,11,16-24H2,1-5H3,(H2,54,70)(H,55,58)(H,56,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t39-,40-,41-,42-,43-,44-,45+,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021996

(Ac-His-Pro-Phe-His-Statine-Leu-Val-NH2 | CHEMBL295...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C47H70N12O9/c1-26(2)16-33(39(61)21-40(62)54-34(17-27(3)4)45(66)58-41(28(5)6)42(48)63)55-44(65)36(19-31-22-49-24-51-31)56-43(64)35(18-30-12-9-8-10-13-30)57-46(67)38-14-11-15-59(38)47(68)37(53-29(7)60)20-32-23-50-25-52-32/h8-10,12-13,22-28,33-39,41,61H,11,14-21H2,1-7H3,(H2,48,63)(H,49,51)(H,50,52)(H,53,60)(H,54,62)(H,55,65)(H,56,64)(H,57,67)(H,58,66)/t33-,34-,35-,36-,37-,38+,39-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022014
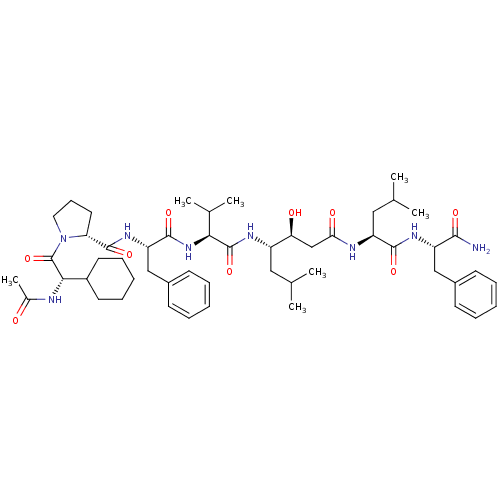
(1-(2-Acetylamino-2-cyclohexyl-acetyl)-pyrrolidine-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@@H](NC(C)=O)C1CCCCC1)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H78N8O9/c1-31(2)26-38(43(62)30-44(63)55-40(27-32(3)4)48(65)57-39(47(53)64)28-35-18-11-8-12-19-35)56-51(68)45(33(5)6)59-49(66)41(29-36-20-13-9-14-21-36)58-50(67)42-24-17-25-60(42)52(69)46(54-34(7)61)37-22-15-10-16-23-37/h8-9,11-14,18-21,31-33,37-43,45-46,62H,10,15-17,22-30H2,1-7H3,(H2,53,64)(H,54,61)(H,55,63)(H,56,68)(H,57,65)(H,58,67)(H,59,66)/t38-,39-,40-,41-,42+,43-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50273160

(IMARIKIREN)Show SMILES COCCCCn1c(nc2ccccc12)C(=O)N(CC(C)C)[C@@H]1CNC[C@@H](C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C27H41N5O4/c1-20(2)19-32(22-16-21(17-28-18-22)26(33)30-11-14-36-15-12-30)27(34)25-29-23-8-4-5-9-24(23)31(25)10-6-7-13-35-3/h4-5,8-9,20-22,28H,6-7,10-19H2,1-3H3/t21-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of renin in rat plasma |
Bioorg Med Chem 26: 3261-3286 (2018)
Article DOI: 10.1016/j.bmc.2018.04.051
BindingDB Entry DOI: 10.7270/Q2VH5RCG |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021997

(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H74N10O9/c1-30(2)21-37(44(63)27-45(64)56-39(22-31(3)4)47(66)58-38(46(52)65)24-34-15-10-8-11-16-34)57-48(67)40(23-32(5)6)59-49(68)41(25-35-17-12-9-13-18-35)60-50(69)43-19-14-20-61(43)51(70)42(55-33(7)62)26-36-28-53-29-54-36/h8-13,15-18,28-32,37-44,63H,14,19-27H2,1-7H3,(H2,52,65)(H,53,54)(H,55,62)(H,56,64)(H,57,67)(H,58,66)(H,59,68)(H,60,69)/t37-,38-,39-,40-,41-,42-,43+,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50367760

(CHEMBL2367544)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H]1CCCN1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H73N13O9/c1-3-35(2)50(56(78)65-43(51(58)73)26-37-17-9-5-10-18-37)69-49(72)30-48(71)42(25-36-15-7-4-8-16-36)64-54(76)45(28-39-31-59-33-62-39)66-53(75)44(27-38-19-11-6-12-20-38)67-55(77)47-22-14-24-70(47)57(79)46(29-40-32-60-34-63-40)68-52(74)41-21-13-23-61-41/h4-12,15-20,31-35,41-48,50,61,71H,3,13-14,21-30H2,1-2H3,(H2,58,73)(H,59,62)(H,60,63)(H,64,76)(H,65,78)(H,66,75)(H,67,77)(H,68,74)(H,69,72)/t35-,41+,42-,43-,44-,45-,46-,47+,48-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50014134
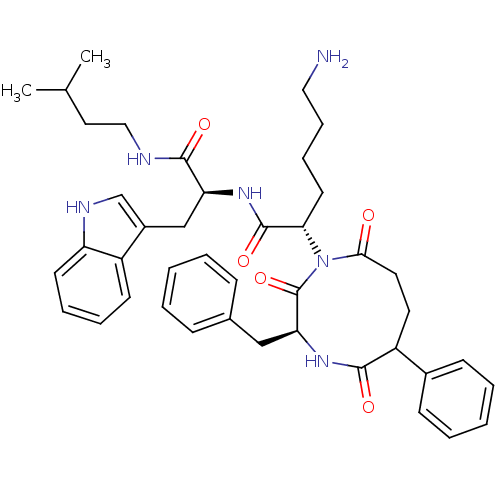
(6-Amino-2-(3-benzyl-2,5,9-trioxo-6-phenyl-[1,4]dia...)Show SMILES CC(C)CCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCCN)N1C(=O)CCC(c2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C1=O Show InChI InChI=1S/C42H52N6O5/c1-28(2)22-24-44-40(51)35(26-31-27-45-34-18-10-9-17-32(31)34)46-41(52)37(19-11-12-23-43)48-38(49)21-20-33(30-15-7-4-8-16-30)39(50)47-36(42(48)53)25-29-13-5-3-6-14-29/h3-10,13-18,27-28,33,35-37,45H,11-12,19-26,43H2,1-2H3,(H,44,51)(H,46,52)(H,47,50)/t33?,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Porcine renin |
J Med Chem 33: 2560-8 (1990)
BindingDB Entry DOI: 10.7270/Q20V8BR8 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022019
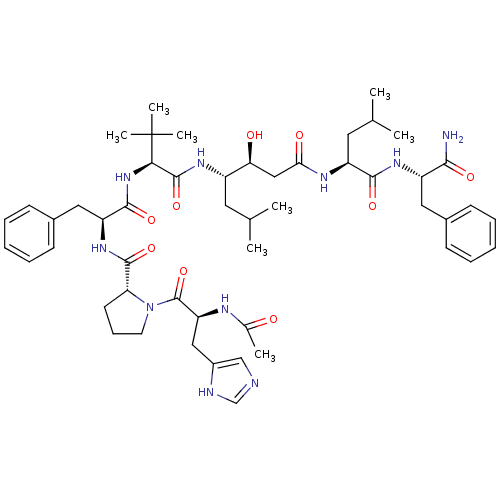
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H74N10O9/c1-30(2)22-36(42(63)27-43(64)56-38(23-31(3)4)46(66)58-37(45(52)65)24-33-16-11-9-12-17-33)57-49(69)44(51(6,7)8)60-47(67)39(25-34-18-13-10-14-19-34)59-48(68)41-20-15-21-61(41)50(70)40(55-32(5)62)26-35-28-53-29-54-35/h9-14,16-19,28-31,36-42,44,63H,15,20-27H2,1-8H3,(H2,52,65)(H,53,54)(H,55,62)(H,56,64)(H,57,69)(H,58,66)(H,59,68)(H,60,67)/t36-,37-,38-,39-,40-,41+,42-,44+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022582
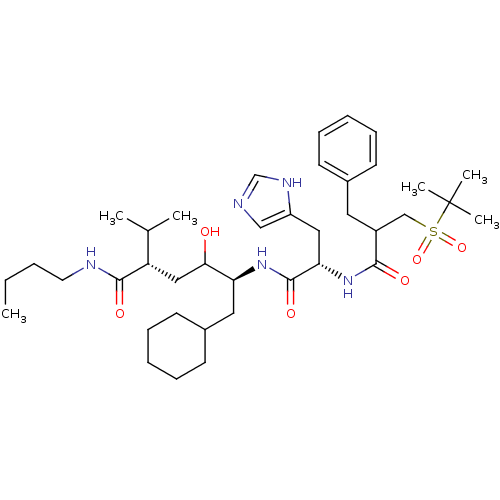
(CHEMBL53139 | Diastereomer-5-[2-[2-Benzyl-3-(2-met...)Show SMILES CCCCNC(=O)[C@@H](CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30?,32-,33-,34-,35?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy Limited
Curated by ChEMBL
| Assay Description
In vitro inhibition of rat plasma renin |
J Med Chem 31: 1839-46 (1988)
BindingDB Entry DOI: 10.7270/Q2Z03744 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50014137

(6-Amino-2-(3-benzyl-8-isobutyl-2,5,9-trioxo-[1,4,7...)Show SMILES CC(C)CCNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCCN)N1C(=O)[C@H](CC(C)C)NCC(=O)N[C@@H](Cc2ccccc2)C1=O Show InChI InChI=1S/C39H55N7O5/c1-25(2)17-19-41-36(48)31(22-28-23-42-30-15-9-8-14-29(28)30)45-37(49)34(16-10-11-18-40)46-38(50)32(20-26(3)4)43-24-35(47)44-33(39(46)51)21-27-12-6-5-7-13-27/h5-9,12-15,23,25-26,31-34,42-43H,10-11,16-22,24,40H2,1-4H3,(H,41,48)(H,44,47)(H,45,49)/t31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against Porcine renin |
J Med Chem 33: 2560-8 (1990)
BindingDB Entry DOI: 10.7270/Q20V8BR8 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024178

(4-(2-{4-[2-(3-Adamantan-1-yl-2-tert-butoxycarbonyl...)Show SMILES COC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CC12CC3CC(CC(C3)C1)C2)NC(=O)OC(C)(C)C |r,TLB:46:47:51:45.44.50,THB:46:45:51:47.52.48,48:47:44:49.51.50,48:49:44:47.52.46| Show InChI InChI=1S/C44H73N7O10/c1-24(2)10-31(35(52)16-37(54)47-26(5)39(56)48-32(11-25(3)4)36(53)17-38(55)60-9)49-40(57)33(15-30-22-45-23-46-30)50-41(58)34(51-42(59)61-43(6,7)8)21-44-18-27-12-28(19-44)14-29(13-27)20-44/h22-29,31-36,52-53H,10-21H2,1-9H3,(H,45,46)(H,47,54)(H,48,56)(H,49,57)(H,50,58)(H,51,59) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against monkey plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50024167

(3-Hydroxy-4-[2-(3-hydroxy-6-methyl-4-{2-[2-(3-meth...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CC(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C39H65N5O9/c1-9-10-16-28(42-39(53)31(41-34(47)19-25(6)7)20-27-14-12-11-13-15-27)38(52)44-29(17-23(2)3)32(45)21-35(48)40-26(8)37(51)43-30(18-24(4)5)33(46)22-36(49)50/h11-15,23-26,28-33,45-46H,9-10,16-22H2,1-8H3,(H,40,48)(H,41,47)(H,42,53)(H,43,51)(H,44,52)(H,49,50) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against rat plasma renin |
J Med Chem 29: 1152-9 (1987)
BindingDB Entry DOI: 10.7270/Q2VH5PD3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































