

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041062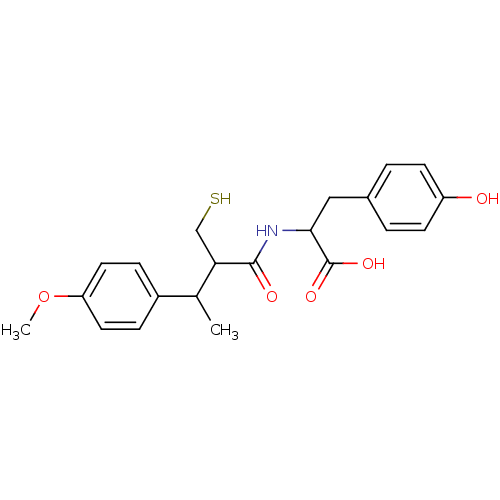 (3-(4-Hydroxy-phenyl)-2-[2-mercaptomethyl-3-(4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041060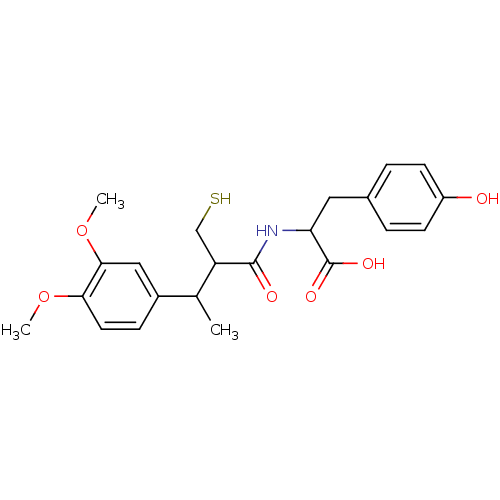 (2-[3-(3,4-Dimethoxy-phenyl)-2-mercaptomethyl-butyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041063 (2-(2-Mercaptomethyl-3-phenyl-butyrylamino)-pentano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041054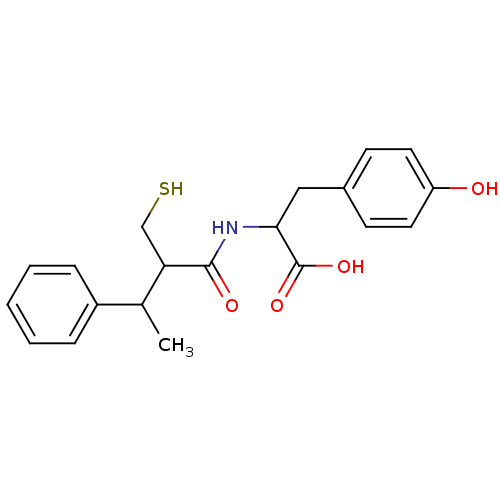 (3-(4-Hydroxy-phenyl)-2-(2-mercaptomethyl-3-phenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041057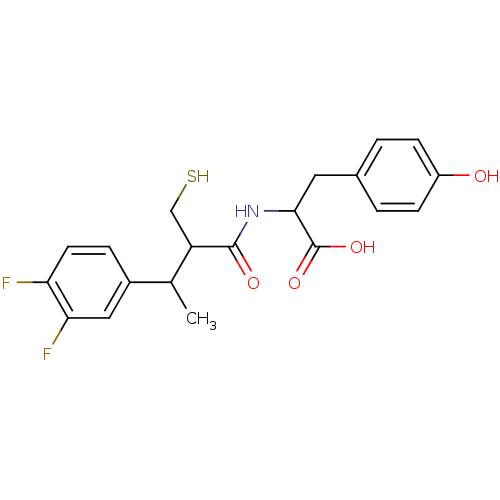 (2-[3-(3,4-Difluoro-phenyl)-2-mercaptomethyl-butyry...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041053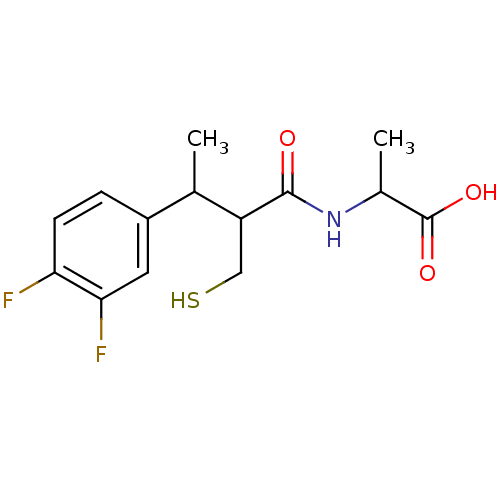 (2-[3-(3,4-Difluoro-phenyl)-2-mercaptomethyl-butyry...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041052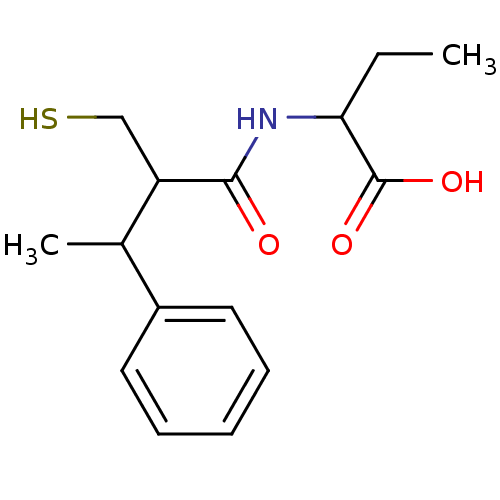 (2-(2-Mercaptomethyl-3-phenyl-butyrylamino)-butyric...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041064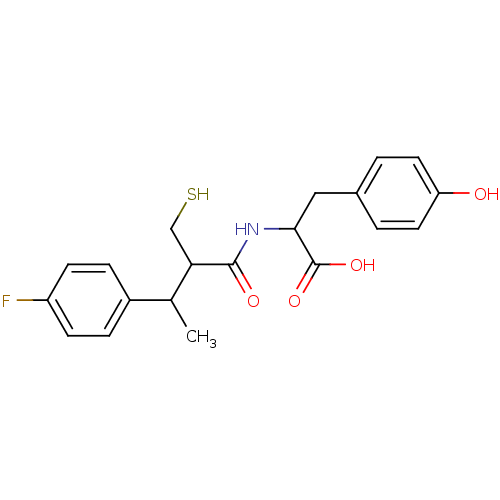 (2-[3-(4-Fluoro-phenyl)-2-mercaptomethyl-butyrylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041061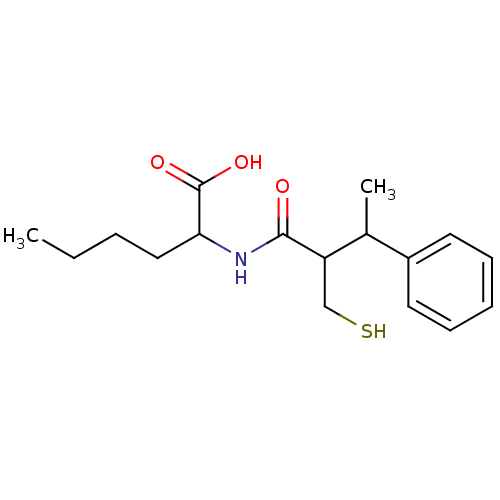 (2-(2-Mercaptomethyl-3-phenyl-butyrylamino)-hexanoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041068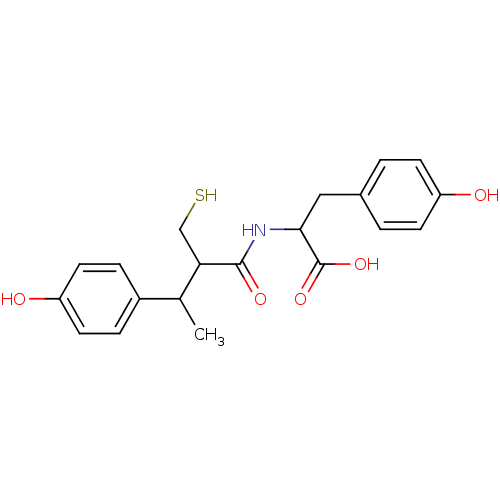 (3-(4-Hydroxy-phenyl)-2-[3-(4-hydroxy-phenyl)-2-mer...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041058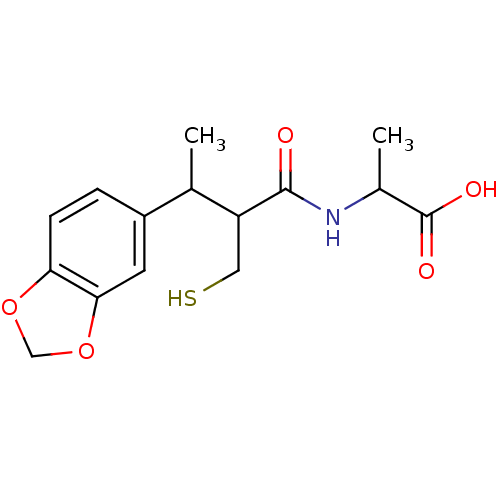 (2-(3-Benzo[1,3]dioxol-5-yl-2-mercaptomethyl-butyry...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041067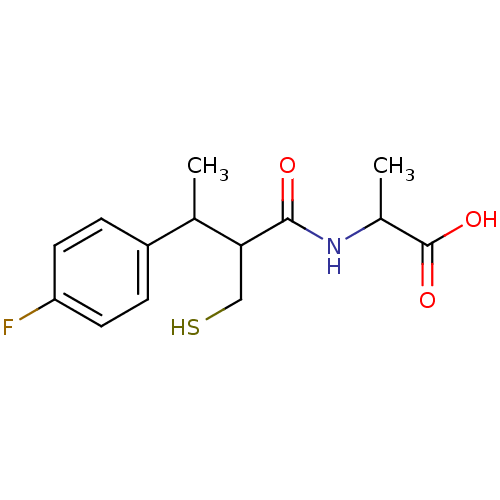 (2-[3-(4-Fluoro-phenyl)-2-mercaptomethyl-butyrylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041055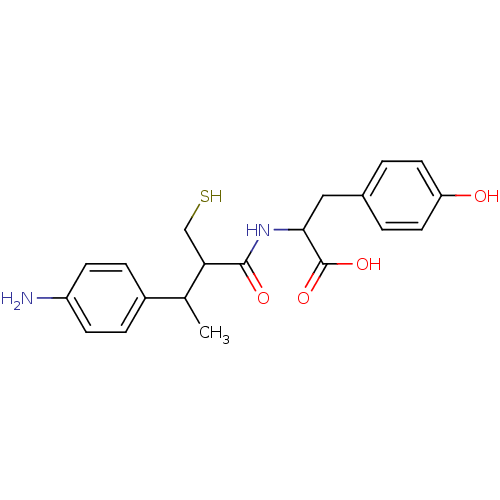 (2-[3-(4-Amino-phenyl)-2-mercaptomethyl-butyrylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50367254 (ENALAPRILAT) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041051 (2-[3-(4-Hydroxy-3-methoxy-phenyl)-2-mercaptomethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041059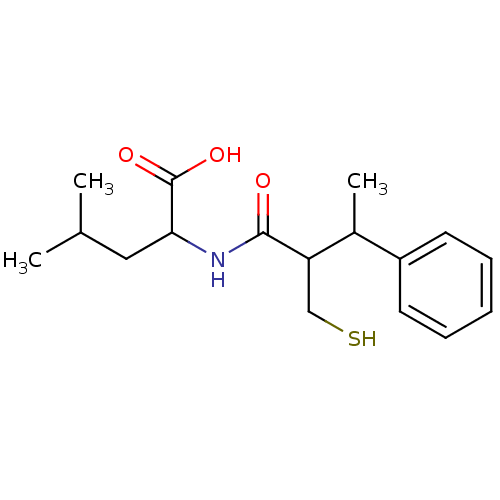 (2-(2-Mercaptomethyl-3-phenyl-butyrylamino)-4-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407297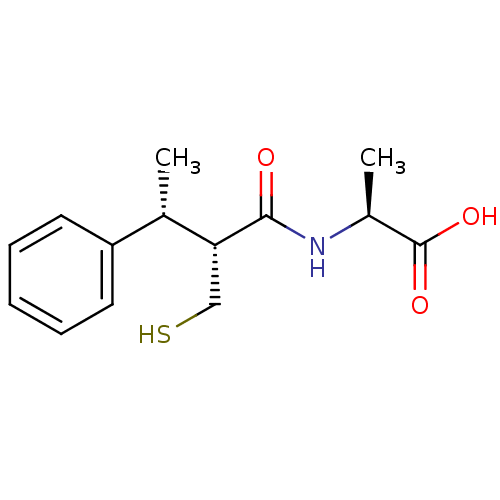 (CHEMBL2052008) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041069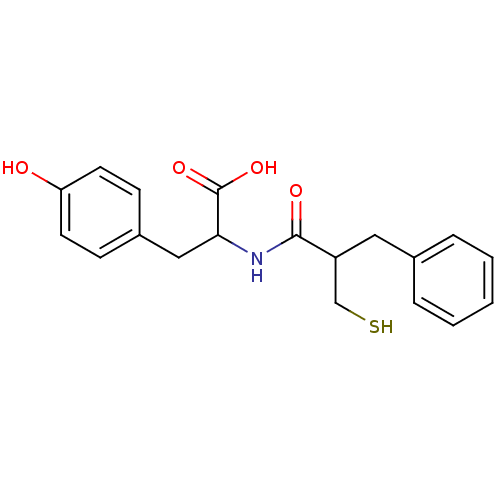 (3-(4-Hydroxy-phenyl)-2-(2-mercaptomethyl-3-phenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041071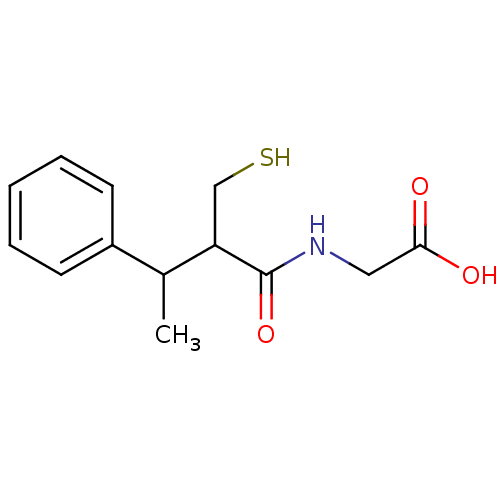 ((2-Mercaptomethyl-3-phenyl-butyrylamino)-acetic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041066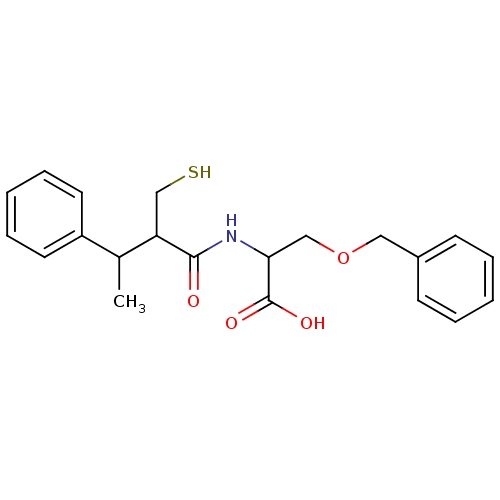 (3-Benzyloxy-2-(2-mercaptomethyl-3-phenyl-butyrylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50242266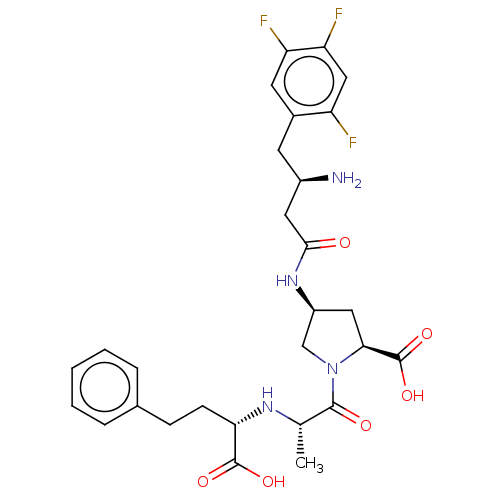 (CHEMBL4067185) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibition of ob/ob mouse plasma ACE | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041065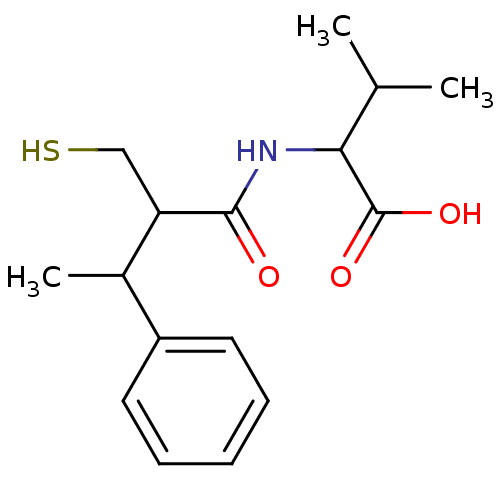 (2-(2-Mercaptomethyl-3-phenyl-butyrylamino)-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50242264 (CHEMBL4086264) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibition of ob/ob mouse plasma ACE | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041070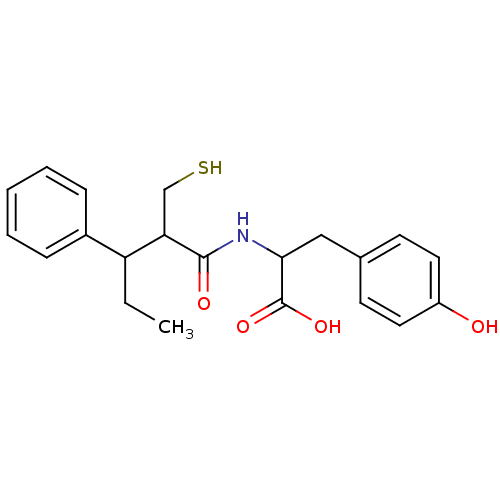 (3-(4-Hydroxy-phenyl)-2-(2-mercaptomethyl-3-phenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041050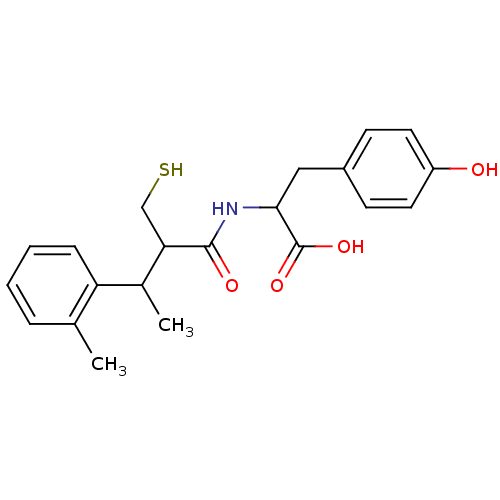 (3-(4-Hydroxy-phenyl)-2-(2-mercaptomethyl-3-o-tolyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50027518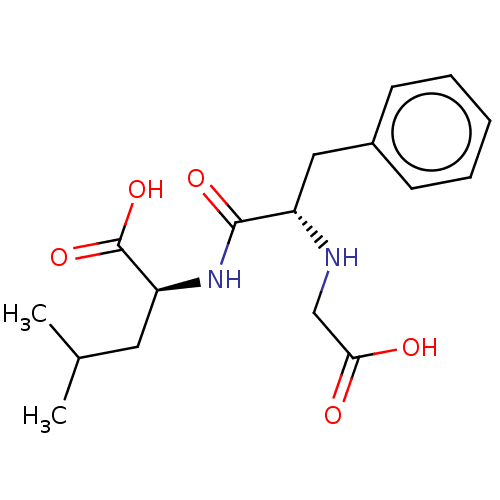 ((2S)-2-[(2R)-2-[(carboxylatomethyl)amino]-3-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50242265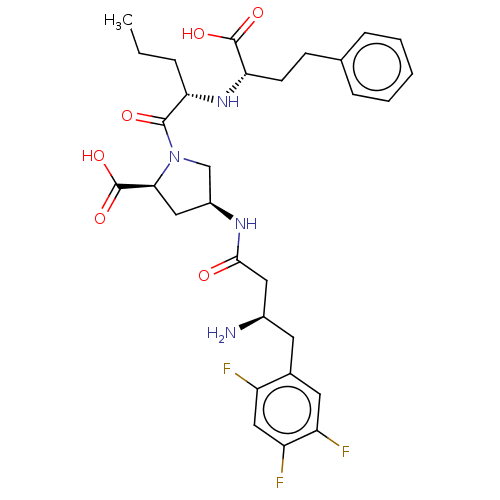 (CHEMBL4078112) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibition of ob/ob mouse plasma ACE | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50242274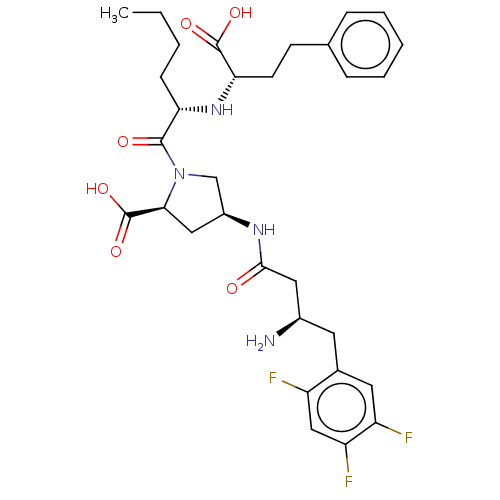 (CHEMBL4090635) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibition of ob/ob mouse plasma ACE | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50452141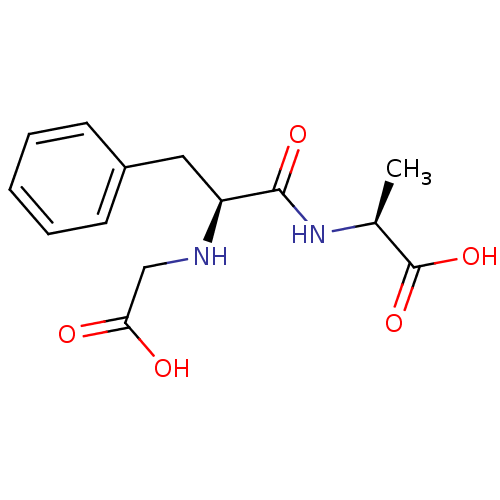 (CHEMBL2372595) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50027514 (2-(2-Carboxymethyl-3-phenyl-propionylamino)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50027513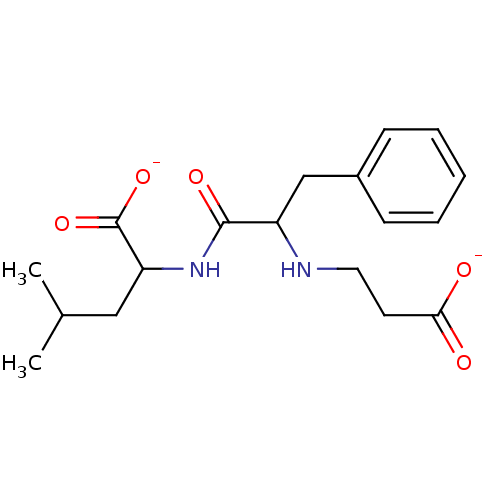 (2-[2-(2-Carboxy-ethylamino)-3-phenyl-propionylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50027516 (2-(2-Amino-3-phenyl-propionylamino)-4-methyl-penta...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against Angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50027522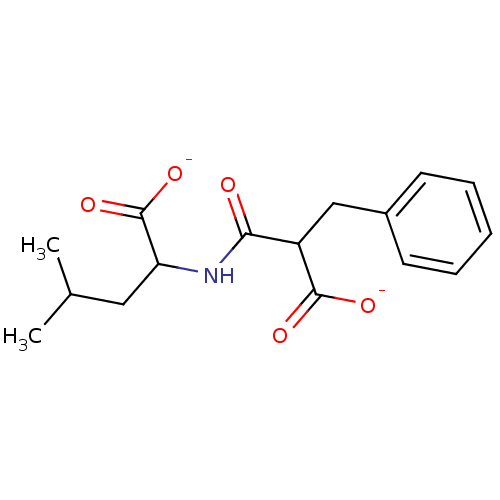 (2-(2-Carboxy-3-phenyl-propionylamino)-4-methyl-pen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against Angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50139892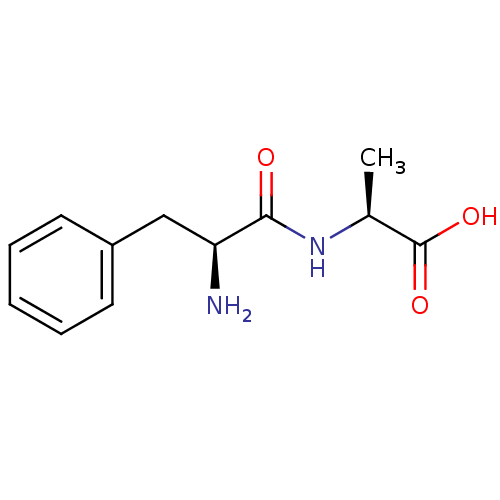 ((S)-2-((S)-2-Amino-3-phenyl-propionylamino)-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% Inhibitory potency against Angiotensin I converting enzyme from mouse striatum. | J Med Chem 26: 60-5 (1983) BindingDB Entry DOI: 10.7270/Q2Q81DP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||