

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50006222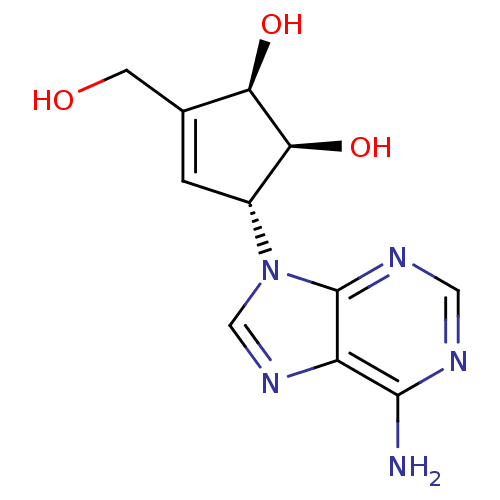 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company Curated by ChEMBL | Assay Description Reversible inhibition of rat liver S- adenosyl-L-homocysteine hydrolase using SAH as substrate by spectrophotometric method | J Med Chem 63: 6315-6386 (2020) Article DOI: 10.1021/acs.jmedchem.9b01877 BindingDB Entry DOI: 10.7270/Q2NG4V6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50046747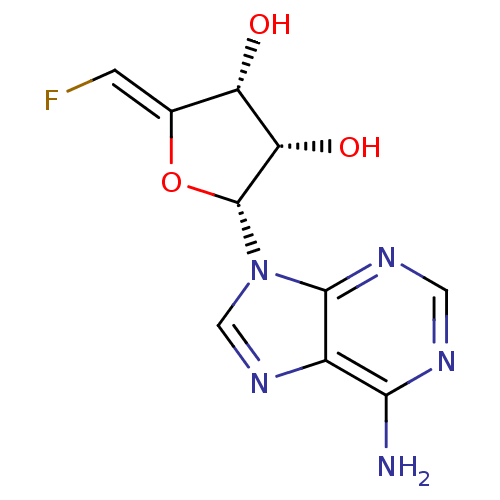 (2-(6-Amino-purin-9-yl)-5-fluoromethylene-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase | J Med Chem 36: 883-7 (1993) BindingDB Entry DOI: 10.7270/Q2HM593F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50051436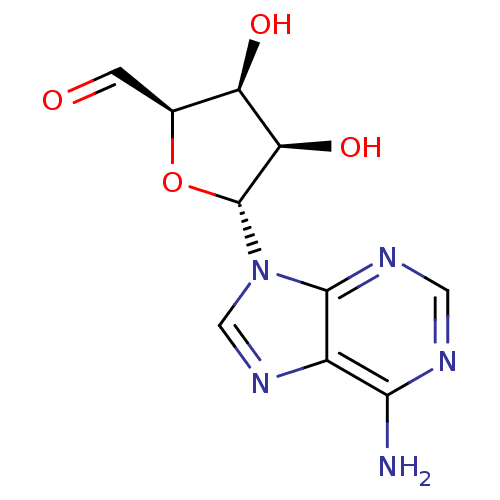 ((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase | J Med Chem 36: 883-7 (1993) BindingDB Entry DOI: 10.7270/Q2HM593F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280299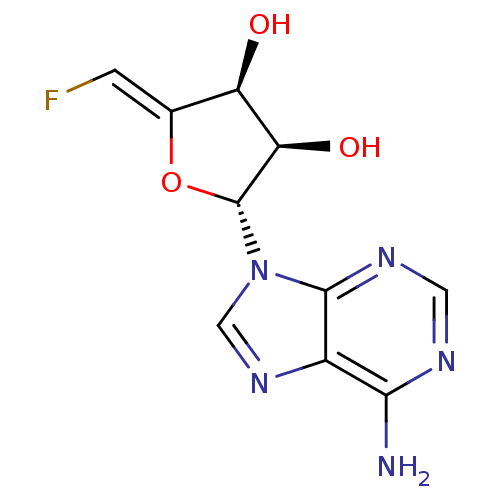 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase | Bioorg Med Chem Lett 2: 1741-1744 (1992) Article DOI: 10.1016/S0960-894X(00)80467-9 BindingDB Entry DOI: 10.7270/Q21Z44B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50046748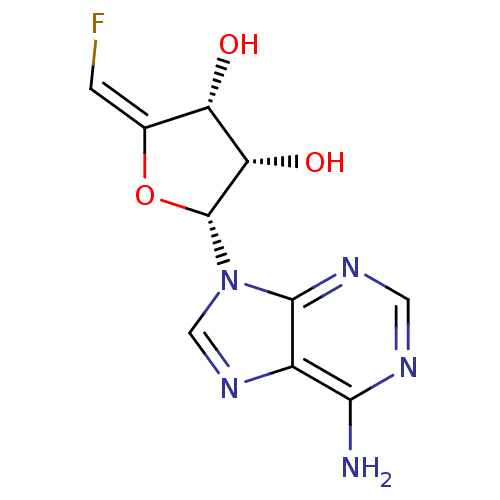 (2-(6-Amino-purin-9-yl)-5-fluoromethylene-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase | J Med Chem 36: 883-7 (1993) BindingDB Entry DOI: 10.7270/Q2HM593F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280301 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase | Bioorg Med Chem Lett 2: 1741-1744 (1992) Article DOI: 10.1016/S0960-894X(00)80467-9 BindingDB Entry DOI: 10.7270/Q21Z44B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50051435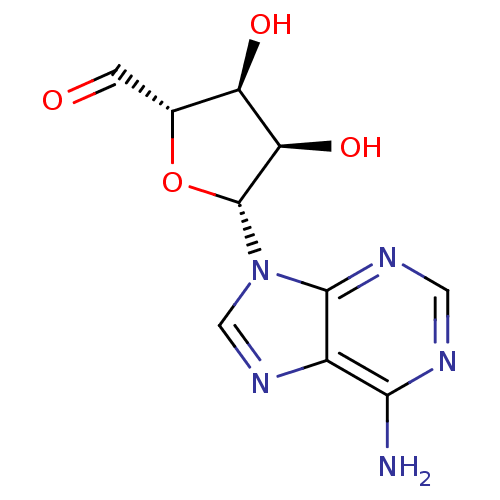 (5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase | J Med Chem 36: 883-7 (1993) BindingDB Entry DOI: 10.7270/Q2HM593F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280300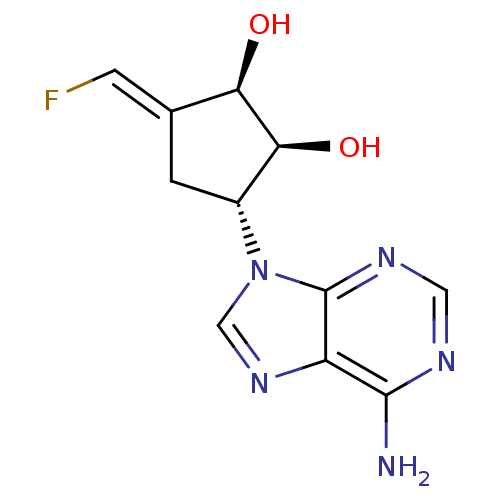 ((1R,2S,3R)-3-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase | Bioorg Med Chem Lett 2: 1741-1744 (1992) Article DOI: 10.1016/S0960-894X(00)80467-9 BindingDB Entry DOI: 10.7270/Q21Z44B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280298 ((1R,2S,3R)-3-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase | Bioorg Med Chem Lett 2: 1741-1744 (1992) Article DOI: 10.1016/S0960-894X(00)80467-9 BindingDB Entry DOI: 10.7270/Q21Z44B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50105900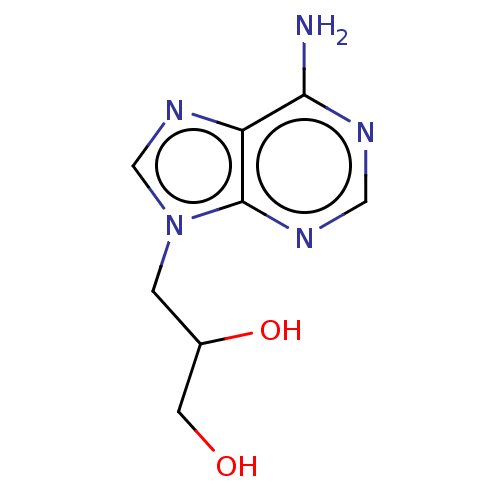 (CHEMBL11390) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Reversible inhibition of rat liver S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem 23: 4952-69 (2015) Article DOI: 10.1016/j.bmc.2015.05.018 BindingDB Entry DOI: 10.7270/Q29888ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280298 ((1R,2S,3R)-3-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for binding affinity of compound against S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 3: 165-168 (1993) Article DOI: 10.1016/S0960-894X(01)80869-6 BindingDB Entry DOI: 10.7270/Q2DR2VDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368169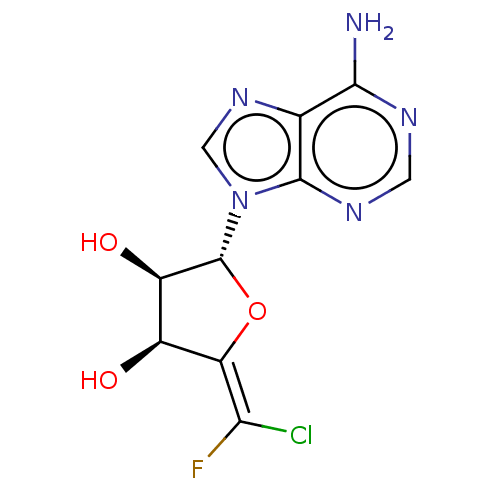 (CHEMBL2368687) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicable | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368167 (CHEMBL3349334 | CHEMBL611905) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicable | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280301 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368170 (CHEMBL2368677) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50406477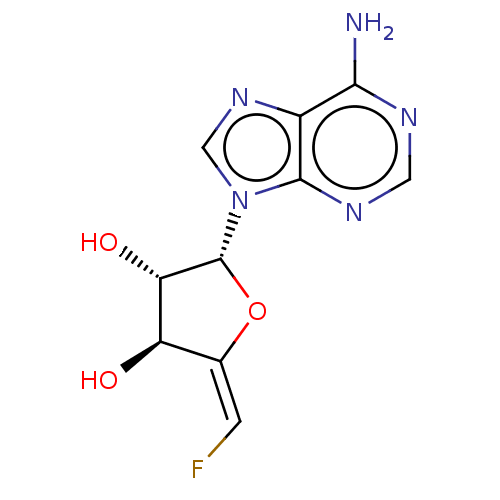 (CHEMBL2051968 | CHEMBL2069133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280299 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as KI values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280300 ((1R,2S,3R)-3-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for binding affinity of compound against S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 3: 165-168 (1993) Article DOI: 10.1016/S0960-894X(01)80869-6 BindingDB Entry DOI: 10.7270/Q2DR2VDD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368168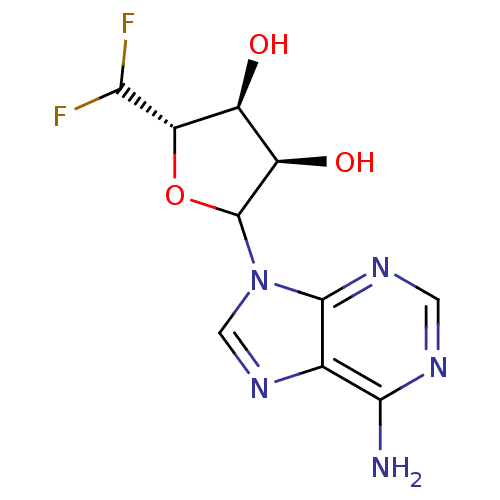 (CHEMBL609353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values. | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50368168 (CHEMBL609353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-adenosyl-L-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50229043 (CHEMBL2051969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Competitive inhibitory activity against rat liver S-Adenosyl-homocysteine hydrolase | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM82524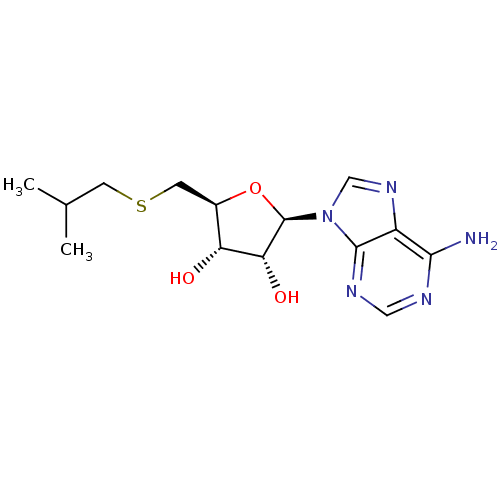 (5'-Deoxy-5'-isobutylthioadenosine | 5'...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition constant for the compound was determined against the recombinant rat liver AdoHyc hydrolase (MV1304/pUCSAH) | Bioorg Med Chem Lett 12: 457-60 (2002) BindingDB Entry DOI: 10.7270/Q2T1546N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50011352 (5-(6-Amino-purin-9-yl)-2-fluoromethylene-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merrell Dow Research Institute Curated by ChEMBL | Assay Description Activity determined in rat liver S-adenosyl-L-homocysteine hydrolase and expressed as Kinactivator values; NA= not applicable | J Med Chem 34: 647-56 (1991) BindingDB Entry DOI: 10.7270/Q25M6695 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50366378 (CHEMBL604650) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 1.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant against rat liver S-adenosyl-L-homocysteine hydrolase | Bioorg Med Chem Lett 5: 1455-1460 (1995) Article DOI: 10.1016/0960-894X(95)00256-S BindingDB Entry DOI: 10.7270/Q2C53MBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50109313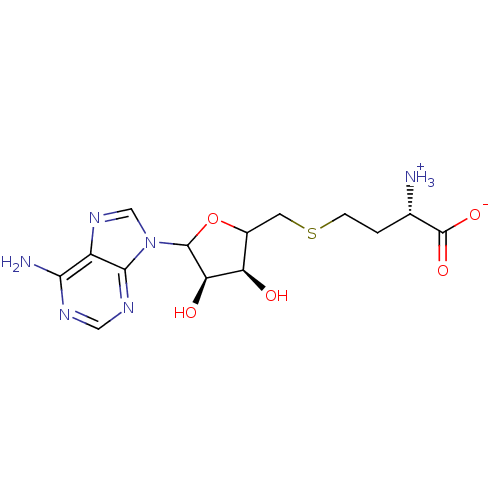 (4-[5-(6-amino-9H-9-purinyl)-3,4-dihydroxy-(3S,4R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.09E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition constant for the compound was determined against the recombinant rat liver AdoHyc hydrolase (MV1304/pUCSAH) | Bioorg Med Chem Lett 12: 457-60 (2002) BindingDB Entry DOI: 10.7270/Q2T1546N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50109313 (4-[5-(6-amino-9H-9-purinyl)-3,4-dihydroxy-(3S,4R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.09E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo Curated by ChEMBL | Assay Description Inhibition constant for the compound was determined against the recombinant rat liver AdoHyc hydrolase (MV1304/pUCSAH) | Bioorg Med Chem Lett 12: 457-60 (2002) BindingDB Entry DOI: 10.7270/Q2T1546N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||