

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380109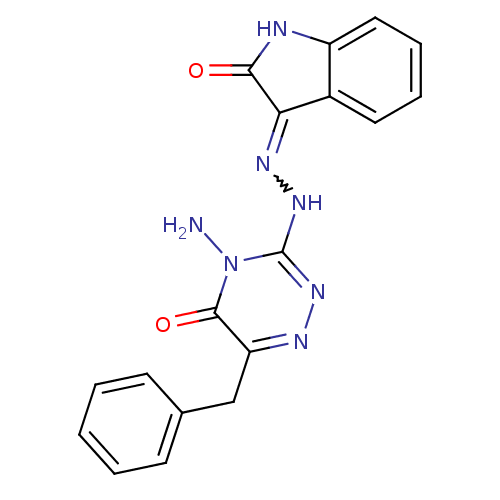 (CHEMBL2013099) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.42 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380110 (CHEMBL2013100) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.53 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380107 (CHEMBL2013097) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.69 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380108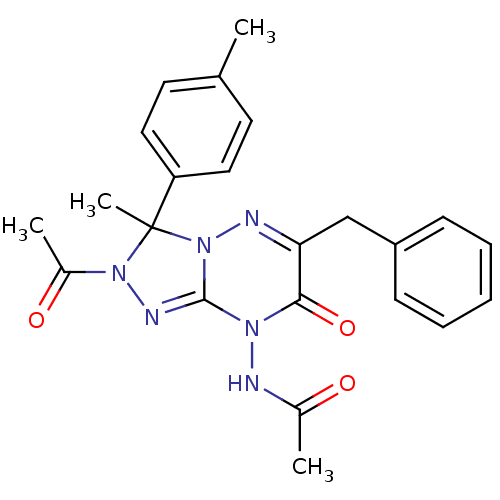 (CHEMBL2013098) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.62 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380111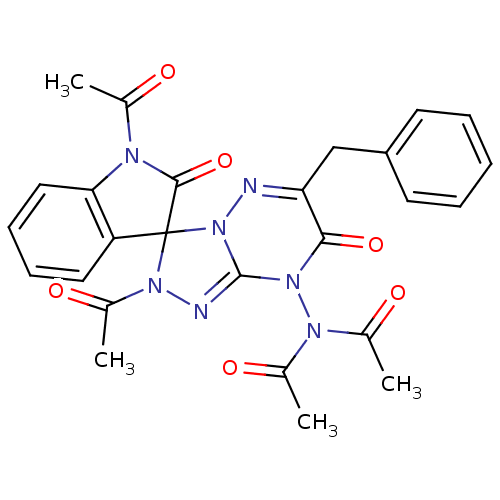 (CHEMBL2013101) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.74 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380106 (CHEMBL2013096) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.36 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380103 (CHEMBL2013093) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.71 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380104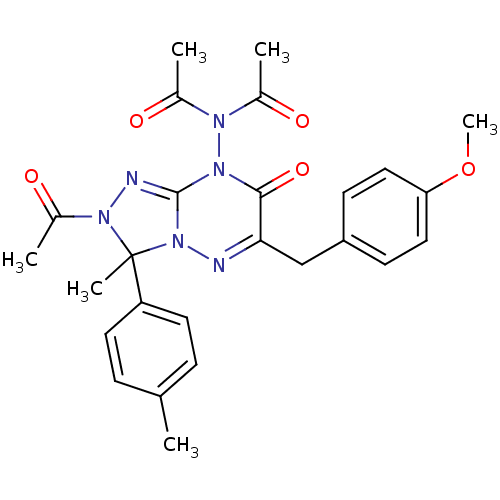 (CHEMBL2013094) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380105 (CHEMBL2013095) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380101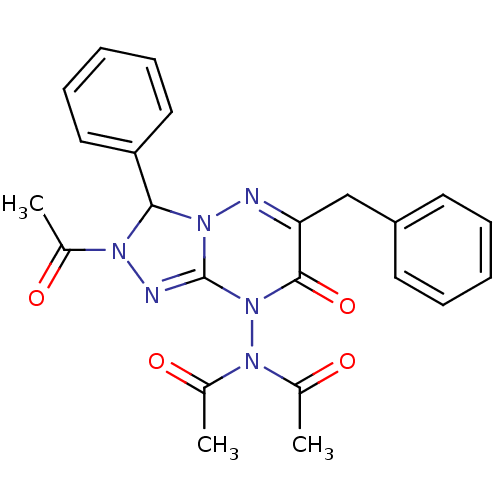 (CHEMBL2013091) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380112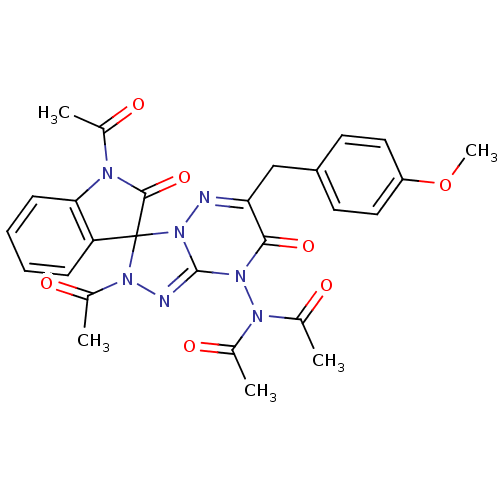 (CHEMBL2013102) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61.4 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50380102 (CHEMBL2013092) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86.7 | n/a | n/a | n/a | n/a | n/a | n/a |
King Abdulaziz University Curated by ChEMBL | Assay Description Inhibition of CYP1A1 activity in rat liver microsomes using benzo[alpha]pyrene as substrate after 10 mins by fluorescence analysis | Bioorg Med Chem 20: 2624-37 (2012) Article DOI: 10.1016/j.bmc.2012.02.041 BindingDB Entry DOI: 10.7270/Q2ZG6T7G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404853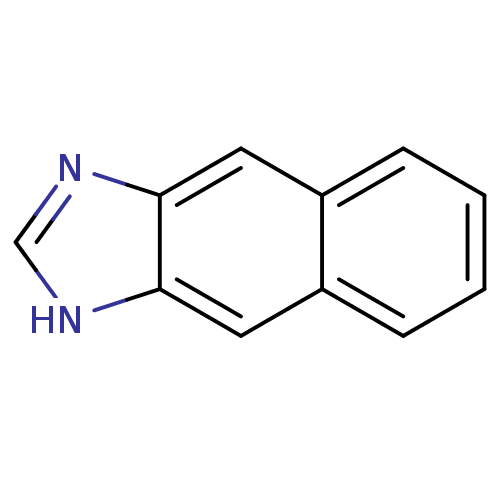 (CHEMBL156313) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effect on Aryl hydrocarbon hydroxylase activity in 3-methylcolanthrene-induced rat liver microsomes at 2.6x10E-4M | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404855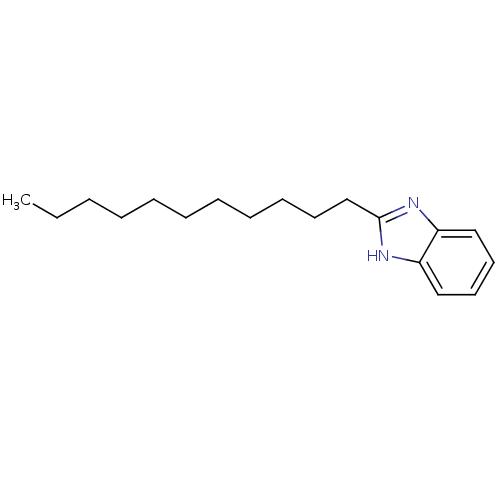 (CHEMBL158007) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404855 (CHEMBL158007) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50017716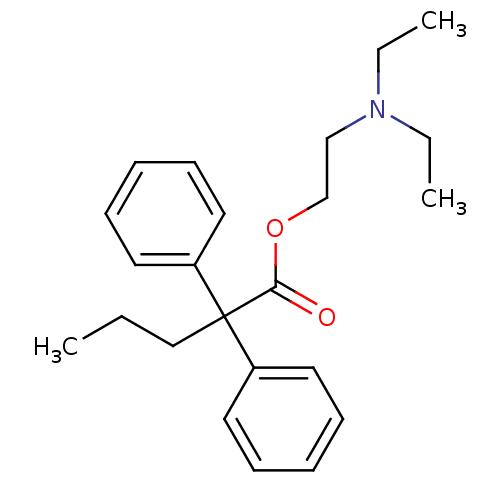 (2,2-Diphenyl-pentanoic acid 2-diethylamino-ethyl e...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404852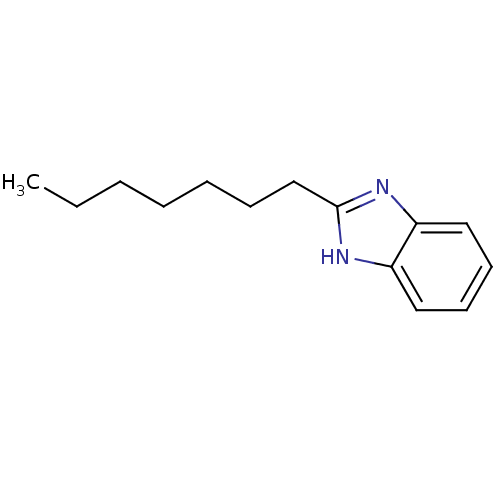 (CHEMBL345675) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404852 (CHEMBL345675) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM24656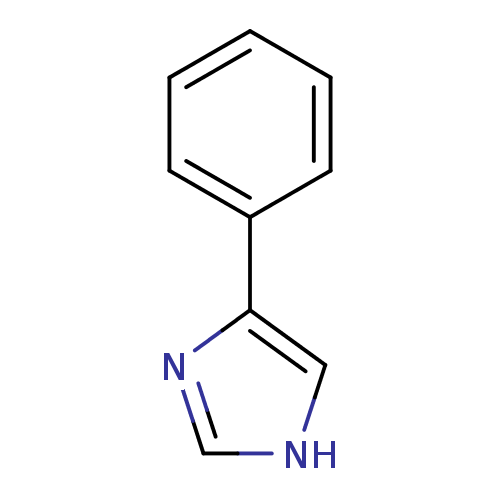 (4-PIM | 4-Phenylimidazole | 4-phenyl-1H-imidazole ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404854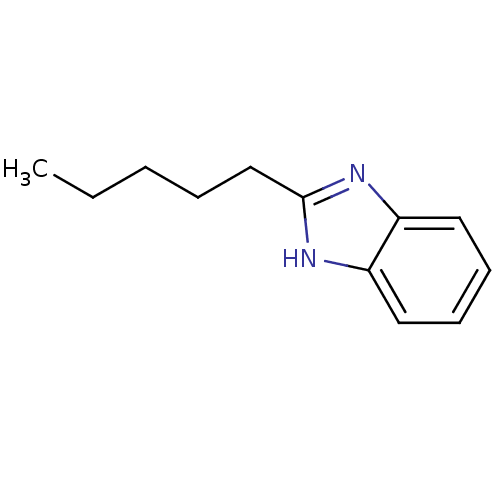 (CHEMBL155867) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404854 (CHEMBL155867) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404853 (CHEMBL156313) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50208872 (5,6-dimethyl-1H-benzimidazole | 5,6-dimethylbenzim...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50208872 (5,6-dimethyl-1H-benzimidazole | 5,6-dimethylbenzim...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 9.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404851 (CHEMBL156439) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404851 (CHEMBL156439) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404856 (CHEMBL156134) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404856 (CHEMBL156134) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404849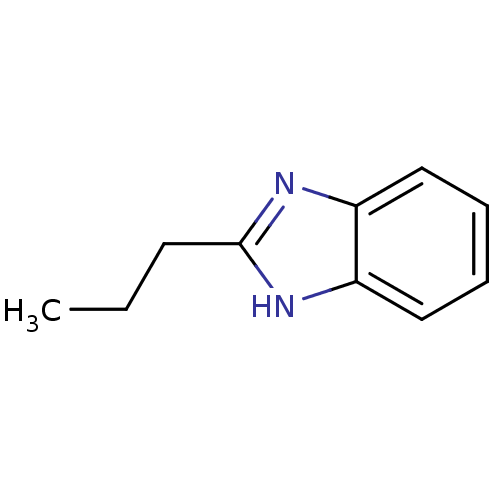 (CHEMBL348508) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404849 (CHEMBL348508) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404858 (CHEMBL351569) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404858 (CHEMBL351569) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404850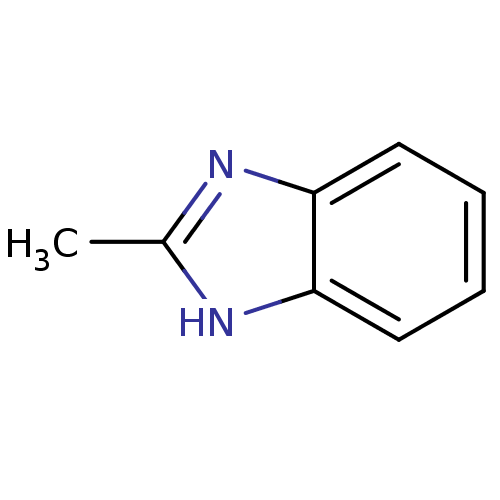 (CHEMBL309135) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 6.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM50404850 (CHEMBL309135) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 6.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM7939 (1H-1,3-benzodiazole | Benzimidazole | Benzimidazol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Rattus norvegicus) | BDBM7939 (1H-1,3-benzodiazole | Benzimidazole | Benzimidazol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PubMed | n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Aryl hydrocarbon hydroxylase in phenobarbitone-treated rats | J Med Chem 25: 622-6 (1982) BindingDB Entry DOI: 10.7270/Q2474C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||