 Found 80 hits of ic50 data for polymerid = 50002231
Found 80 hits of ic50 data for polymerid = 50002231 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50090529
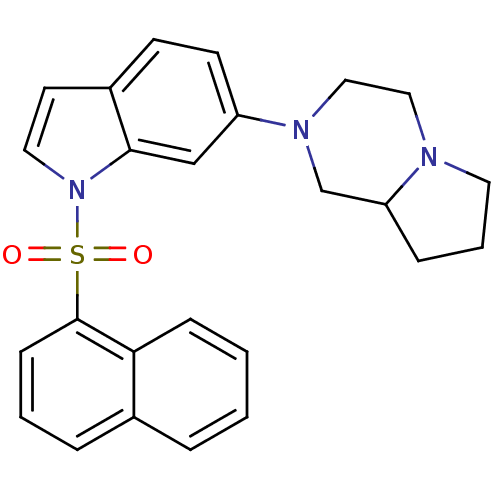
(2-[1-(Naphthalene-1-sulfonyl)-1H-indol-6-yl]-octah...)Show SMILES O=S(=O)(c1cccc2ccccc12)n1ccc2ccc(cc12)N1CCN2CCCC2C1 Show InChI InChI=1S/C25H25N3O2S/c29-31(30,25-9-3-6-19-5-1-2-8-23(19)25)28-14-12-20-10-11-21(17-24(20)28)27-16-15-26-13-4-7-22(26)18-27/h1-3,5-6,8-12,14,17,22H,4,7,13,15-16,18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Allelix Corp.
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound evaluated in adenylyl cyclase assay |
Bioorg Med Chem Lett 10: 1719-21 (2000)
BindingDB Entry DOI: 10.7270/Q2BP021W |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128187
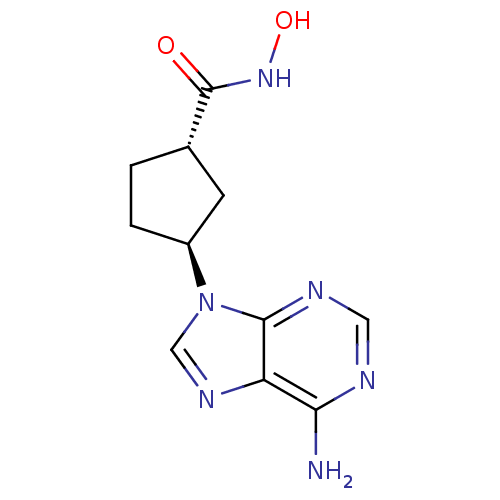
((1R,3R)-3-(6-Amino-purin-9-yl)-cyclopentanecarboxy...)Show InChI InChI=1S/C11H14N6O2/c12-9-8-10(14-4-13-9)17(5-15-8)7-2-1-6(3-7)11(18)16-19/h4-7,19H,1-3H2,(H,16,18)(H2,12,13,14)/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128192
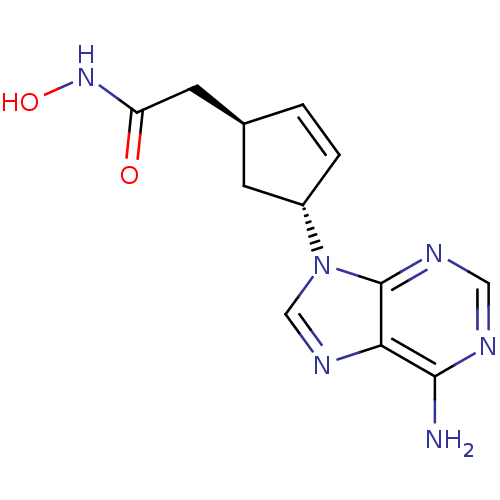
((1S,3R)-2-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@@H](CC(=O)NO)C=C1 |c:20| Show InChI InChI=1S/C12H14N6O2/c13-11-10-12(15-5-14-11)18(6-16-10)8-2-1-7(3-8)4-9(19)17-20/h1-2,5-8,20H,3-4H2,(H,17,19)(H2,13,14,15)/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128210
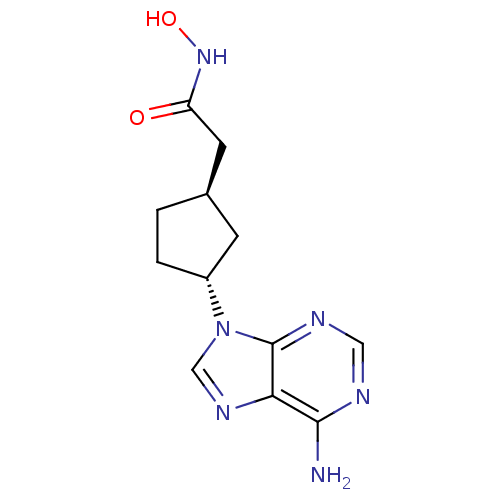
((1R,3R)-2-[3-(6-Amino-purin-9-yl)-cyclopentyl]-N-h...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1CC[C@@H](CC(=O)NO)C1 Show InChI InChI=1S/C12H16N6O2/c13-11-10-12(15-5-14-11)18(6-16-10)8-2-1-7(3-8)4-9(19)17-20/h5-8,20H,1-4H2,(H,17,19)(H2,13,14,15)/t7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128207
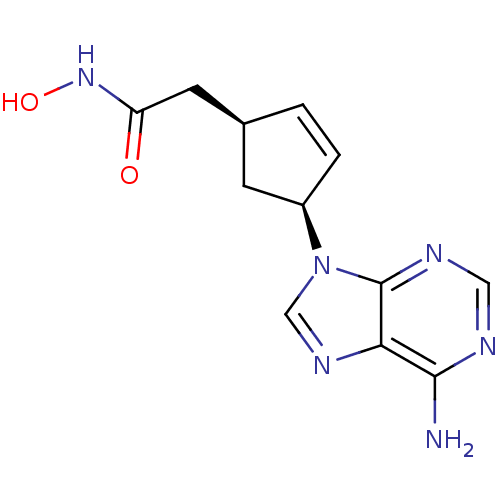
((1S,3S)-2-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@@H](CC(=O)NO)C=C1 |c:20| Show InChI InChI=1S/C12H14N6O2/c13-11-10-12(15-5-14-11)18(6-16-10)8-2-1-7(3-8)4-9(19)17-20/h1-2,5-8,20H,3-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128209
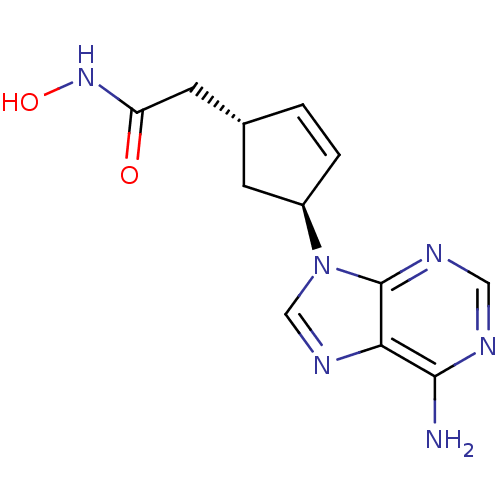
((1R,3S)-2-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@H](CC(=O)NO)C=C1 |c:20| Show InChI InChI=1S/C12H14N6O2/c13-11-10-12(15-5-14-11)18(6-16-10)8-2-1-7(3-8)4-9(19)17-20/h1-2,5-8,20H,3-4H2,(H,17,19)(H2,13,14,15)/t7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128203
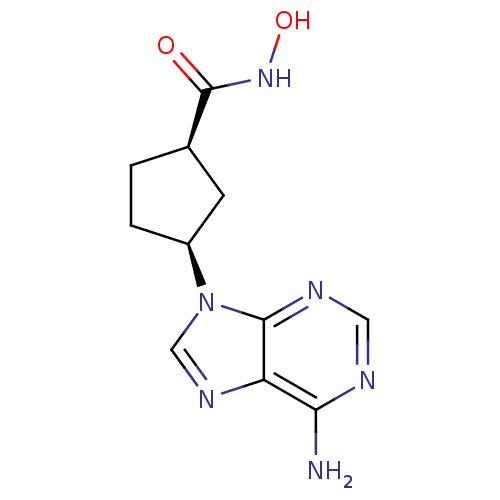
((1R,3S)-3-(6-Amino-purin-9-yl)-cyclopentanecarboxy...)Show InChI InChI=1S/C11H14N6O2/c12-9-8-10(14-4-13-9)17(5-15-8)7-2-1-6(3-7)11(18)16-19/h4-7,19H,1-3H2,(H,16,18)(H2,12,13,14)/t6-,7+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128199
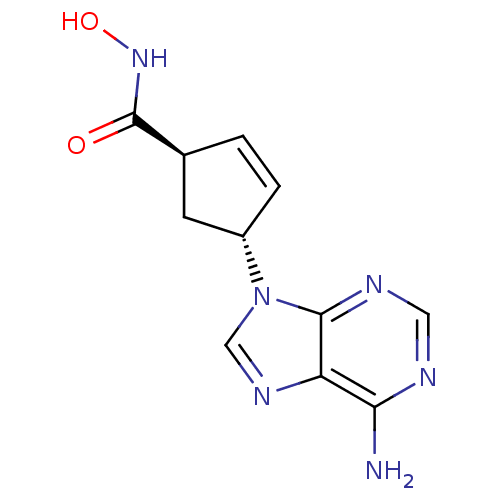
((1S,3S)-4-(6-Amino-purin-9-yl)-cyclopent-2-enecarb...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](C=C1)C(=O)NO |c:15| Show InChI InChI=1S/C11H12N6O2/c12-9-8-10(14-4-13-9)17(5-15-8)7-2-1-6(3-7)11(18)16-19/h1-2,4-7,19H,3H2,(H,16,18)(H2,12,13,14)/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119826
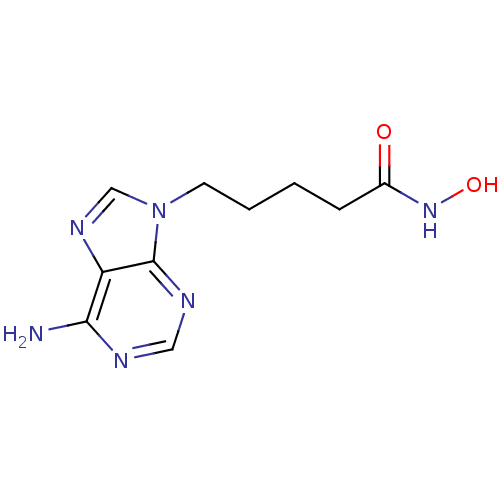
(5-(6-Amino-purin-9-yl)-pentanoic acid hydroxyamide...)Show InChI InChI=1S/C10H14N6O2/c11-9-8-10(13-5-12-9)16(6-14-8)4-2-1-3-7(17)15-18/h5-6,18H,1-4H2,(H,15,17)(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119826

(5-(6-Amino-purin-9-yl)-pentanoic acid hydroxyamide...)Show InChI InChI=1S/C10H14N6O2/c11-9-8-10(13-5-12-9)16(6-14-8)4-2-1-3-7(17)15-18/h5-6,18H,1-4H2,(H,15,17)(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119826

(5-(6-Amino-purin-9-yl)-pentanoic acid hydroxyamide...)Show InChI InChI=1S/C10H14N6O2/c11-9-8-10(13-5-12-9)16(6-14-8)4-2-1-3-7(17)15-18/h5-6,18H,1-4H2,(H,15,17)(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cells |
Bioorg Med Chem Lett 12: 3085-8 (2002)
BindingDB Entry DOI: 10.7270/Q2XW4J5B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119832
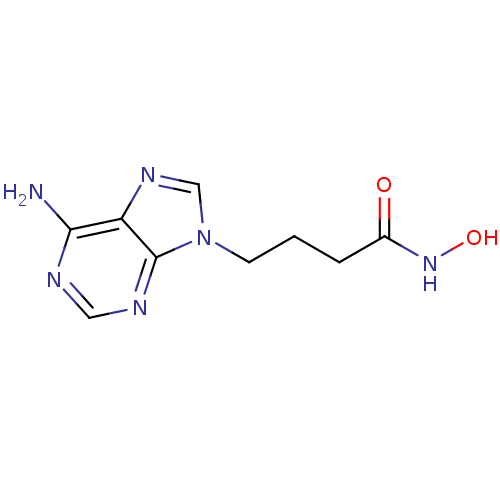
(4-(6-Amino-purin-9-yl)-N-hydroxy-butyramide | CHEM...)Show InChI InChI=1S/C9H12N6O2/c10-8-7-9(12-4-11-8)15(5-13-7)3-1-2-6(16)14-17/h4-5,17H,1-3H2,(H,14,16)(H2,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119832

(4-(6-Amino-purin-9-yl)-N-hydroxy-butyramide | CHEM...)Show InChI InChI=1S/C9H12N6O2/c10-8-7-9(12-4-11-8)15(5-13-7)3-1-2-6(16)14-17/h4-5,17H,1-3H2,(H,14,16)(H2,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cells |
Bioorg Med Chem Lett 12: 3085-8 (2002)
BindingDB Entry DOI: 10.7270/Q2XW4J5B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119832

(4-(6-Amino-purin-9-yl)-N-hydroxy-butyramide | CHEM...)Show InChI InChI=1S/C9H12N6O2/c10-8-7-9(12-4-11-8)15(5-13-7)3-1-2-6(16)14-17/h4-5,17H,1-3H2,(H,14,16)(H2,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128188
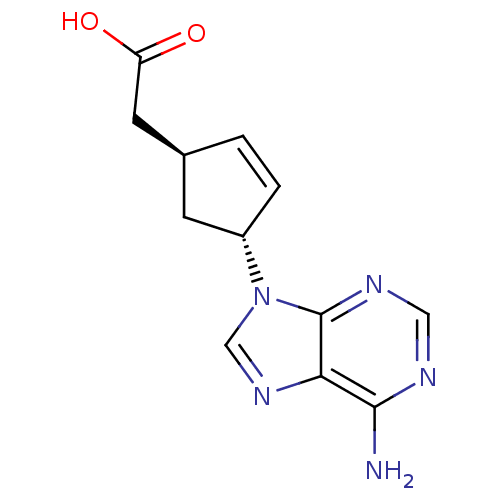
((1S,3R)-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl]-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@@H](CC(O)=O)C=C1 |c:19| Show InChI InChI=1S/C12H13N5O2/c13-11-10-12(15-5-14-11)17(6-16-10)8-2-1-7(3-8)4-9(18)19/h1-2,5-8H,3-4H2,(H,18,19)(H2,13,14,15)/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128206
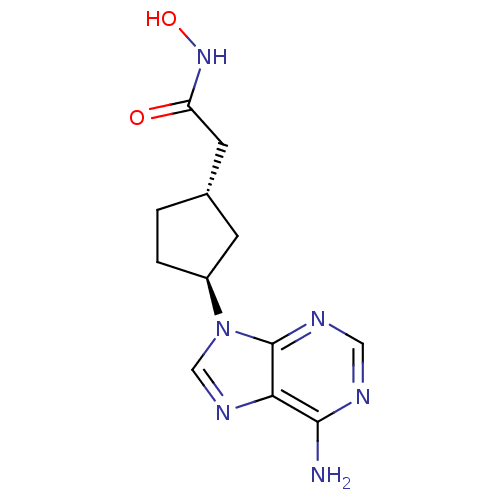
((1S,3S)-2-[3-(6-Amino-purin-9-yl)-cyclopentyl]-N-h...)Show InChI InChI=1S/C12H16N6O2/c13-11-10-12(15-5-14-11)18(6-16-10)8-2-1-7(3-8)4-9(19)17-20/h5-8,20H,1-4H2,(H,17,19)(H2,13,14,15)/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128191
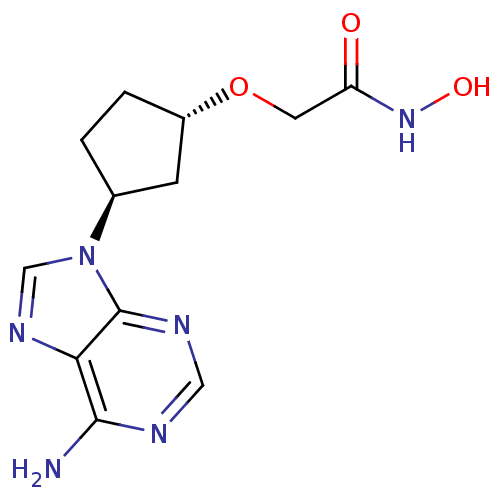
(2-[3-(6-Amino-purin-9-yl)-cyclopentyloxy]-N-hydrox...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1CC[C@@H](C1)OCC(=O)NO Show InChI InChI=1S/C12H16N6O3/c13-11-10-12(15-5-14-11)18(6-16-10)7-1-2-8(3-7)21-4-9(19)17-20/h5-8,20H,1-4H2,(H,17,19)(H2,13,14,15)/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119858
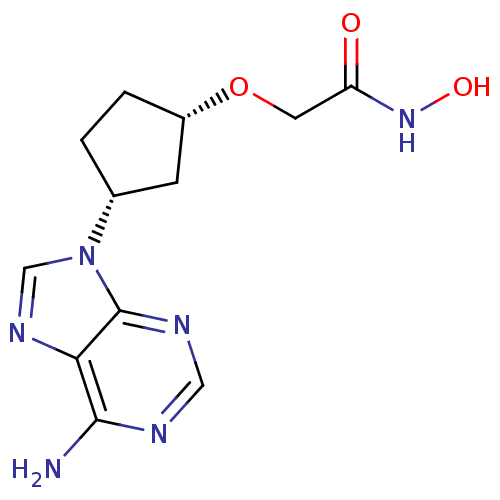
(2-[(S)-3-((S)-6-Amino-purin-9-yl)-cyclopentyloxy]-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1CC[C@@H](C1)OCC(=O)NO Show InChI InChI=1S/C12H16N6O3/c13-11-10-12(15-5-14-11)18(6-16-10)7-1-2-8(3-7)21-4-9(19)17-20/h5-8,20H,1-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119858

(2-[(S)-3-((S)-6-Amino-purin-9-yl)-cyclopentyloxy]-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1CC[C@@H](C1)OCC(=O)NO Show InChI InChI=1S/C12H16N6O3/c13-11-10-12(15-5-14-11)18(6-16-10)7-1-2-8(3-7)21-4-9(19)17-20/h5-8,20H,1-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119855
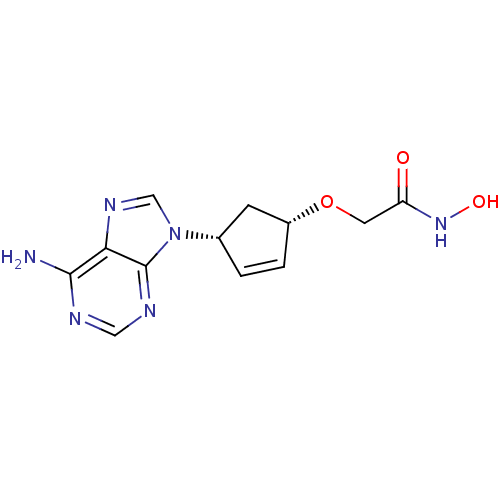
(2-{[(1S,4R)-4-(6-amino-9H-purin-9-yl)cyclopent-2-e...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](OCC(=O)NO)C=C1 |c:21| Show InChI InChI=1S/C12H14N6O3/c13-11-10-12(15-5-14-11)18(6-16-10)7-1-2-8(3-7)21-4-9(19)17-20/h1-2,5-8,20H,3-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119855

(2-{[(1S,4R)-4-(6-amino-9H-purin-9-yl)cyclopent-2-e...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](OCC(=O)NO)C=C1 |c:21| Show InChI InChI=1S/C12H14N6O3/c13-11-10-12(15-5-14-11)18(6-16-10)7-1-2-8(3-7)21-4-9(19)17-20/h1-2,5-8,20H,3-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128205
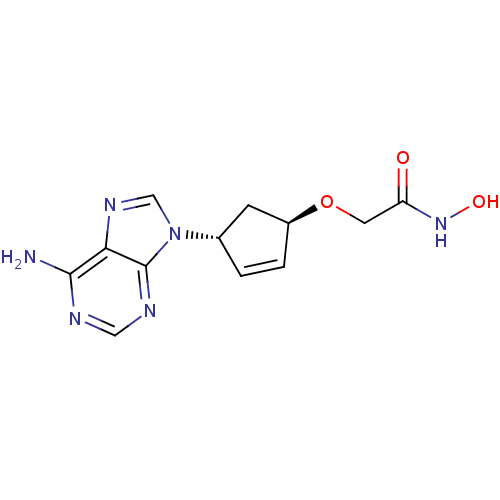
(2-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyloxy]-N-h...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@@H](OCC(=O)NO)C=C1 |c:21| Show InChI InChI=1S/C12H14N6O3/c13-11-10-12(15-5-14-11)18(6-16-10)7-1-2-8(3-7)21-4-9(19)17-20/h1-2,5-8,20H,3-4H2,(H,17,19)(H2,13,14,15)/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119844
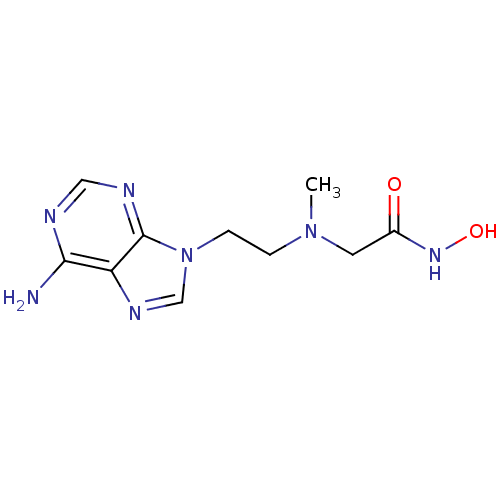
(2-{[2-(6-Amino-purin-9-yl)-ethyl]-methyl-amino}-N-...)Show InChI InChI=1S/C10H15N7O2/c1-16(4-7(18)15-19)2-3-17-6-14-8-9(11)12-5-13-10(8)17/h5-6,19H,2-4H2,1H3,(H,15,18)(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cells |
Bioorg Med Chem Lett 12: 3085-8 (2002)
BindingDB Entry DOI: 10.7270/Q2XW4J5B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128204
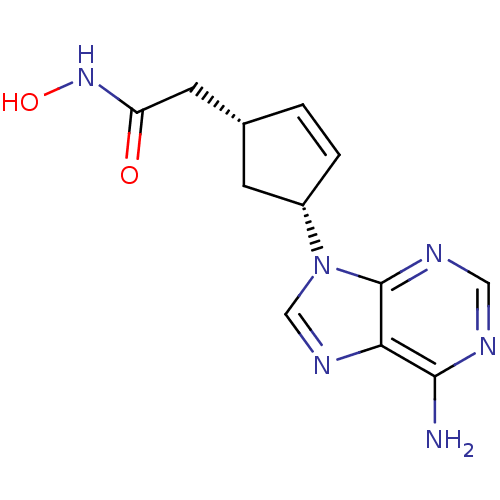
((1R,3R)-2-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](CC(=O)NO)C=C1 |c:20| Show InChI InChI=1S/C12H14N6O2/c13-11-10-12(15-5-14-11)18(6-16-10)8-2-1-7(3-8)4-9(19)17-20/h1-2,5-8,20H,3-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128202
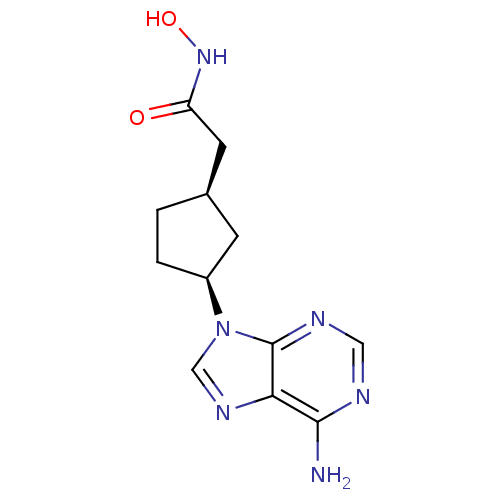
((1R,3S)-2-[3-(6-Amino-purin-9-yl)-cyclopentyl]-N-h...)Show InChI InChI=1S/C12H16N6O2/c13-11-10-12(15-5-14-11)18(6-16-10)8-2-1-7(3-8)4-9(19)17-20/h5-8,20H,1-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128195
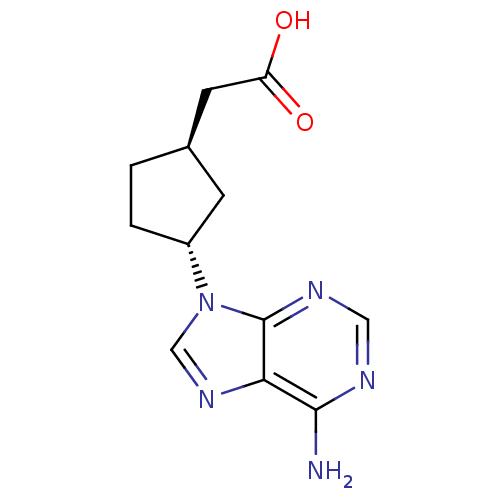
((1R,3R)-[3-(6-Amino-purin-9-yl)-cyclopentyl]-aceti...)Show InChI InChI=1S/C12H15N5O2/c13-11-10-12(15-5-14-11)17(6-16-10)8-2-1-7(3-8)4-9(18)19/h5-8H,1-4H2,(H,18,19)(H2,13,14,15)/t7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119842
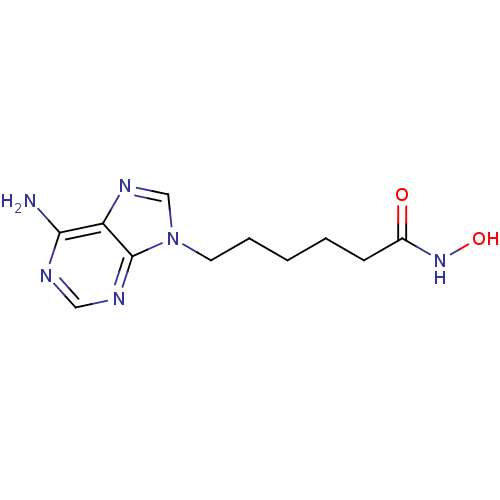
(6-(6-Amino-purin-9-yl)-hexanoic acid hydroxyamide ...)Show InChI InChI=1S/C11H16N6O2/c12-10-9-11(14-6-13-10)17(7-15-9)5-3-1-2-4-8(18)16-19/h6-7,19H,1-5H2,(H,16,18)(H2,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119842

(6-(6-Amino-purin-9-yl)-hexanoic acid hydroxyamide ...)Show InChI InChI=1S/C11H16N6O2/c12-10-9-11(14-6-13-10)17(7-15-9)5-3-1-2-4-8(18)16-19/h6-7,19H,1-5H2,(H,16,18)(H2,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cells |
Bioorg Med Chem Lett 12: 3085-8 (2002)
BindingDB Entry DOI: 10.7270/Q2XW4J5B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119842

(6-(6-Amino-purin-9-yl)-hexanoic acid hydroxyamide ...)Show InChI InChI=1S/C11H16N6O2/c12-10-9-11(14-6-13-10)17(7-15-9)5-3-1-2-4-8(18)16-19/h6-7,19H,1-5H2,(H,16,18)(H2,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128197
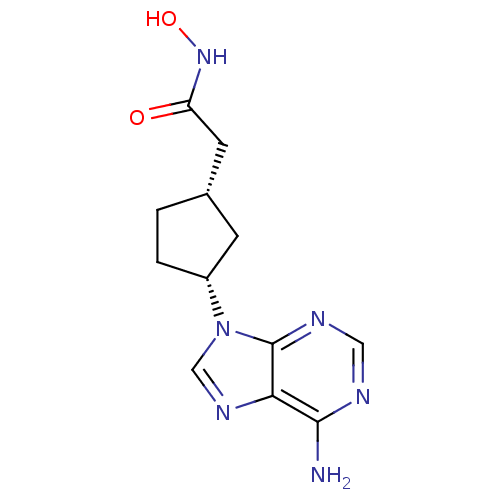
((1S,3R)-2-[3-(6-Amino-purin-9-yl)-cyclopentyl]-N-h...)Show InChI InChI=1S/C12H16N6O2/c13-11-10-12(15-5-14-11)18(6-16-10)8-2-1-7(3-8)4-9(19)17-20/h5-8,20H,1-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128201
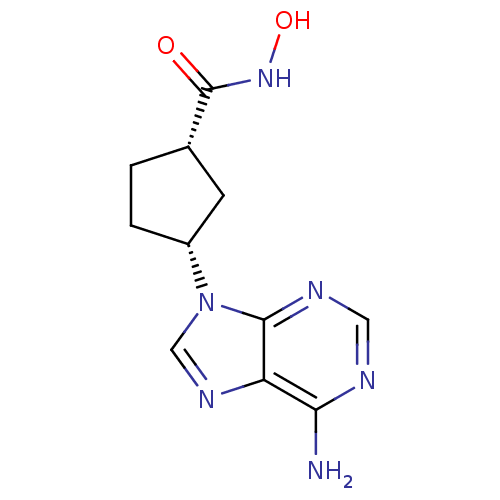
((1S,3R)-3-(6-Amino-purin-9-yl)-cyclopentanecarboxy...)Show InChI InChI=1S/C11H14N6O2/c12-9-8-10(14-4-13-9)17(5-15-8)7-2-1-6(3-7)11(18)16-19/h4-7,19H,1-3H2,(H,16,18)(H2,12,13,14)/t6-,7+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119853
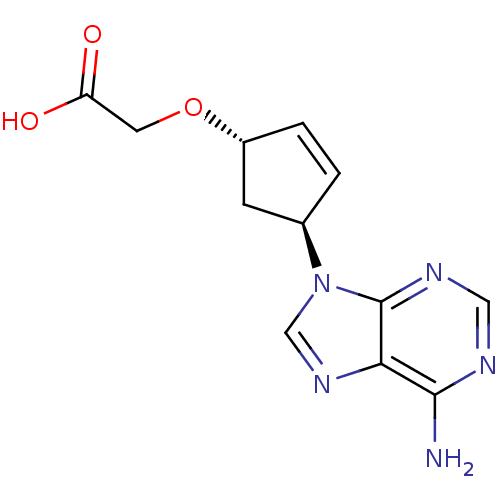
(2-{[(1S,4S)-4-(6-amino-9H-purin-9-yl)cyclopent-2-e...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@H](OCC(O)=O)C=C1 |c:20| Show InChI InChI=1S/C12H13N5O3/c13-11-10-12(15-5-14-11)17(6-16-10)7-1-2-8(3-7)20-4-9(18)19/h1-2,5-8H,3-4H2,(H,18,19)(H2,13,14,15)/t7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128190
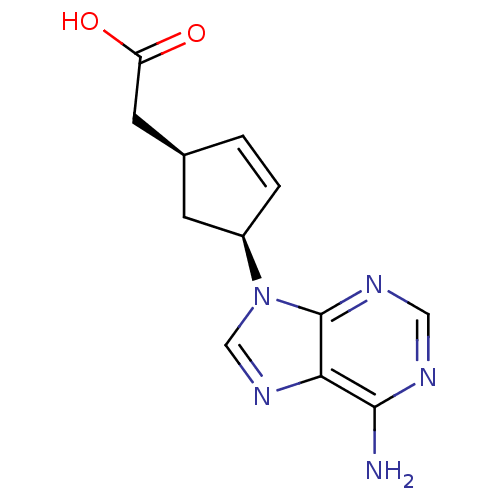
((1S,3S)-[4-(6-Amino-purin-9-yl)-cyclopent-2-enyl]-...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@@H](CC(O)=O)C=C1 |c:19| Show InChI InChI=1S/C12H13N5O2/c13-11-10-12(15-5-14-11)17(6-16-10)8-2-1-7(3-8)4-9(18)19/h1-2,5-8H,3-4H2,(H,18,19)(H2,13,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128186
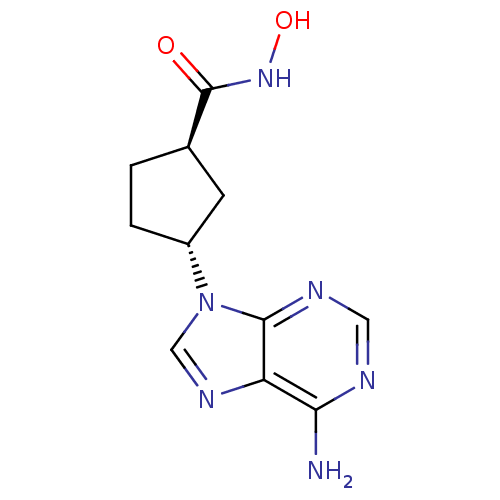
((1S,3S)-3-(6-Amino-purin-9-yl)-cyclopentanecarboxy...)Show InChI InChI=1S/C11H14N6O2/c12-9-8-10(14-4-13-9)17(5-15-8)7-2-1-6(3-7)11(18)16-19/h4-7,19H,1-3H2,(H,16,18)(H2,12,13,14)/t6-,7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119860
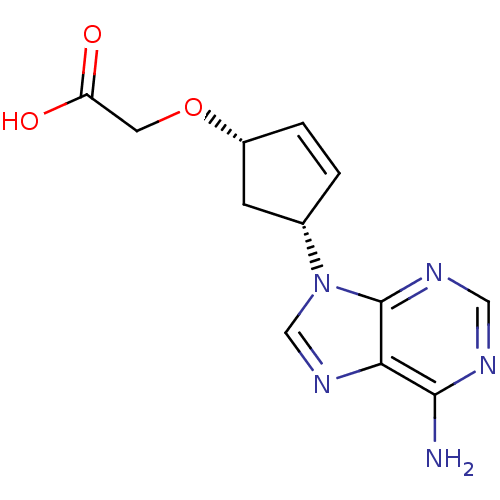
(2-{[(1S,4R)-4-(6-amino-9H-purin-9-yl)cyclopent-2-e...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@H](OCC(O)=O)C=C1 |c:20| Show InChI InChI=1S/C12H13N5O3/c13-11-10-12(15-5-14-11)17(6-16-10)7-1-2-8(3-7)20-4-9(18)19/h1-2,5-8H,3-4H2,(H,18,19)(H2,13,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119857
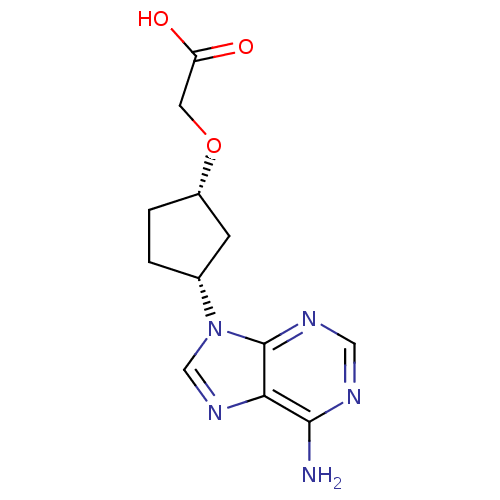
(CHEMBL108067 | [(S)-3-((S)-6-Amino-purin-9-yl)-cyc...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1CC[C@@H](C1)OCC(O)=O Show InChI InChI=1S/C12H15N5O3/c13-11-10-12(15-5-14-11)17(6-16-10)7-1-2-8(3-7)20-4-9(18)19/h5-8H,1-4H2,(H,18,19)(H2,13,14,15)/t7-,8+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119821
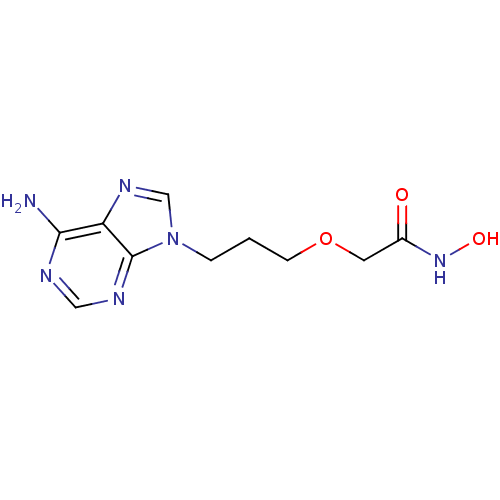
(2-[3-(6-Amino-purin-9-yl)-propoxy]-N-hydroxy-aceta...)Show InChI InChI=1S/C10H14N6O3/c11-9-8-10(13-5-12-9)16(6-14-8)2-1-3-19-4-7(17)15-18/h5-6,18H,1-4H2,(H,15,17)(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119821

(2-[3-(6-Amino-purin-9-yl)-propoxy]-N-hydroxy-aceta...)Show InChI InChI=1S/C10H14N6O3/c11-9-8-10(13-5-12-9)16(6-14-8)2-1-3-19-4-7(17)15-18/h5-6,18H,1-4H2,(H,15,17)(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cells |
Bioorg Med Chem Lett 12: 3085-8 (2002)
BindingDB Entry DOI: 10.7270/Q2XW4J5B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119854
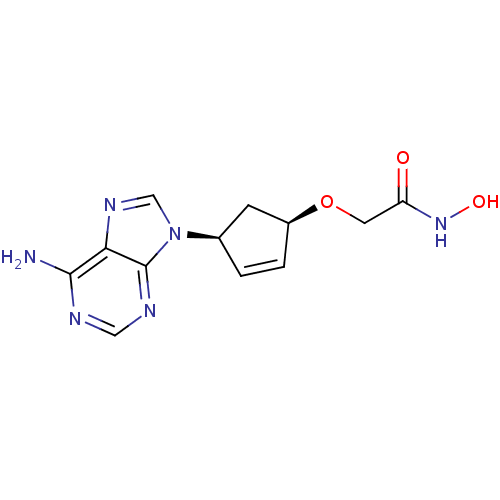
(2-{[(1R,4S)-4-(6-amino-9H-purin-9-yl)cyclopent-2-e...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@@H](OCC(=O)NO)C=C1 |c:21| Show InChI InChI=1S/C12H14N6O3/c13-11-10-12(15-5-14-11)18(6-16-10)7-1-2-8(3-7)21-4-9(19)17-20/h1-2,5-8,20H,3-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119861
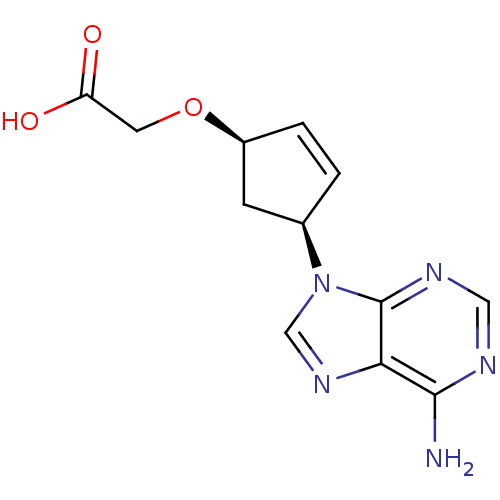
(2-{[(1R,4S)-4-(6-amino-9H-purin-9-yl)cyclopent-2-e...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@@H](OCC(O)=O)C=C1 |c:20| Show InChI InChI=1S/C12H13N5O3/c13-11-10-12(15-5-14-11)17(6-16-10)7-1-2-8(3-7)20-4-9(18)19/h1-2,5-8H,3-4H2,(H,18,19)(H2,13,14,15)/t7-,8+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128194
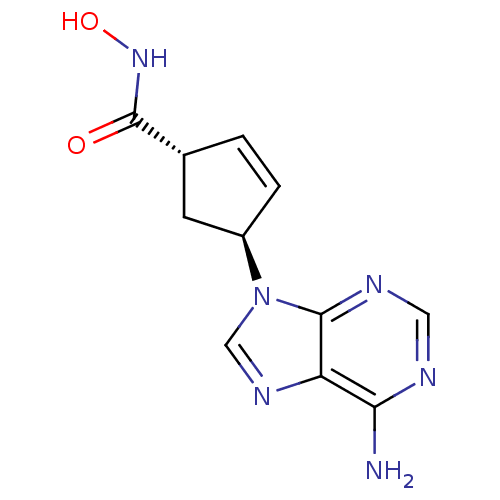
((1R,3R)-4-(6-Amino-purin-9-yl)-cyclopent-2-enecarb...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@@H](C=C1)C(=O)NO |c:15| Show InChI InChI=1S/C11H12N6O2/c12-9-8-10(14-4-13-9)17(5-15-8)7-2-1-6(3-7)11(18)16-19/h1-2,4-7,19H,3H2,(H,16,18)(H2,12,13,14)/t6-,7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128189
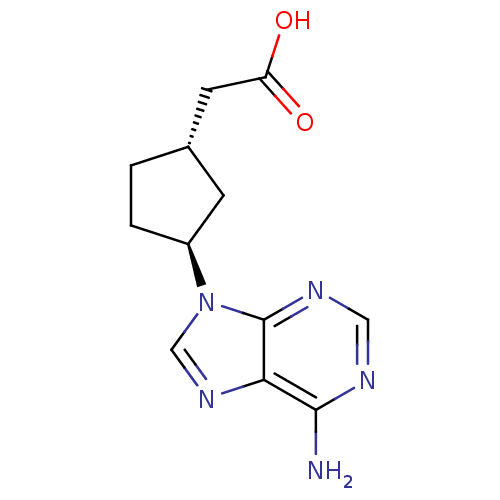
((1S,3S)-[3-(6-Amino-purin-9-yl)-cyclopentyl]-aceti...)Show InChI InChI=1S/C12H15N5O2/c13-11-10-12(15-5-14-11)17(6-16-10)8-2-1-7(3-8)4-9(18)19/h5-8H,1-4H2,(H,18,19)(H2,13,14,15)/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119854

(2-{[(1R,4S)-4-(6-amino-9H-purin-9-yl)cyclopent-2-e...)Show SMILES Nc1ncnc2n(cnc12)[C@H]1C[C@@H](OCC(=O)NO)C=C1 |c:21| Show InChI InChI=1S/C12H14N6O3/c13-11-10-12(15-5-14-11)18(6-16-10)7-1-2-8(3-7)21-4-9(19)17-20/h1-2,5-8,20H,3-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119851
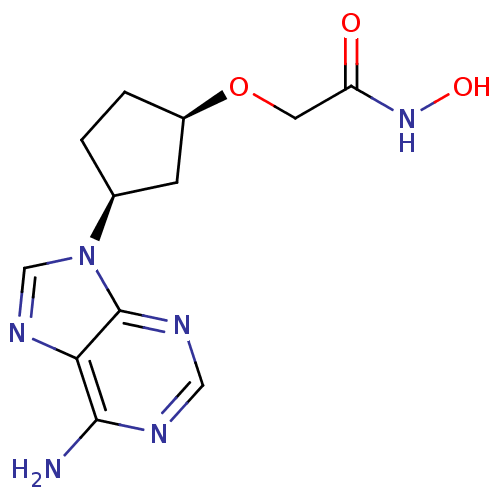
(2-[(R)-3-((R)-6-Amino-purin-9-yl)-cyclopentyloxy]-...)Show InChI InChI=1S/C12H16N6O3/c13-11-10-12(15-5-14-11)18(6-16-10)7-1-2-8(3-7)21-4-9(19)17-20/h5-8,20H,1-4H2,(H,17,19)(H2,13,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119829
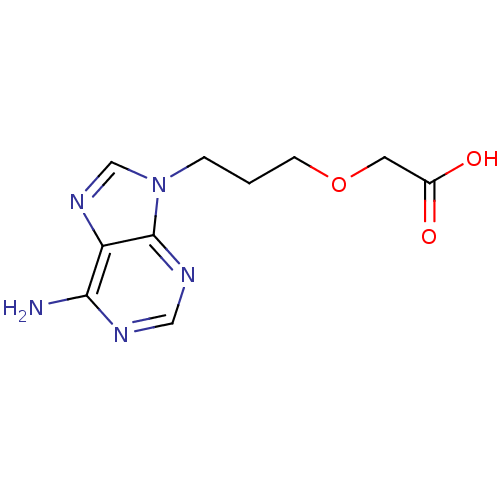
(CHEMBL104969 | [3-(6-Amino-purin-9-yl)-propoxy]-ac...)Show InChI InChI=1S/C10H13N5O3/c11-9-8-10(13-5-12-9)15(6-14-8)2-1-3-18-4-7(16)17/h5-6H,1-4H2,(H,16,17)(H2,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human adenylate cyclase 5 expressed in HEK293 cells |
Bioorg Med Chem Lett 12: 3085-8 (2002)
BindingDB Entry DOI: 10.7270/Q2XW4J5B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50128211
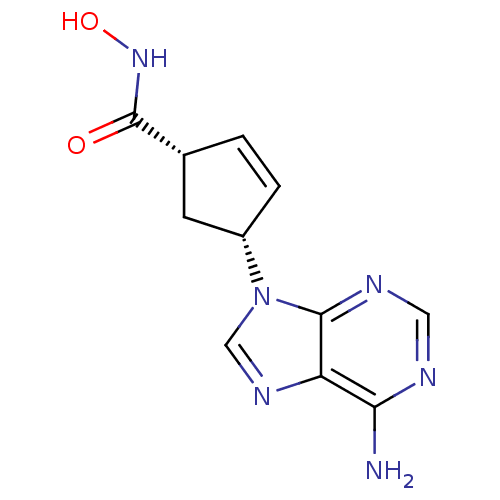
((1S,3R)-4-(6-Amino-purin-9-yl)-cyclopent-2-enecarb...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@@H](C=C1)C(=O)NO |c:15| Show InChI InChI=1S/C11H12N6O2/c12-9-8-10(14-4-13-9)17(5-15-8)7-2-1-6(3-7)11(18)16-19/h1-2,4-7,19H,3H2,(H,16,18)(H2,12,13,14)/t6-,7+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human adenylate cyclase 5 expressed in HEK293 cells. |
J Med Chem 46: 2177-86 (2003)
Article DOI: 10.1021/jm0205604
BindingDB Entry DOI: 10.7270/Q2M32V44 |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119856

(CHEMBL106689 | [(R)-3-((R)-6-Amino-purin-9-yl)-cyc...)Show InChI InChI=1S/C12H15N5O3/c13-11-10-12(15-5-14-11)17(6-16-10)7-1-2-8(3-7)20-4-9(18)19/h5-8H,1-4H2,(H,18,19)(H2,13,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119859

(2-{[(1R,3R)-3-(6-amino-9H-purin-9-yl)cyclopentyl]o...)Show InChI InChI=1S/C12H15N5O3/c13-11-10-12(15-5-14-11)17(6-16-10)7-1-2-8(3-7)20-4-9(18)19/h5-8H,1-4H2,(H,18,19)(H2,13,14,15)/t7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119850
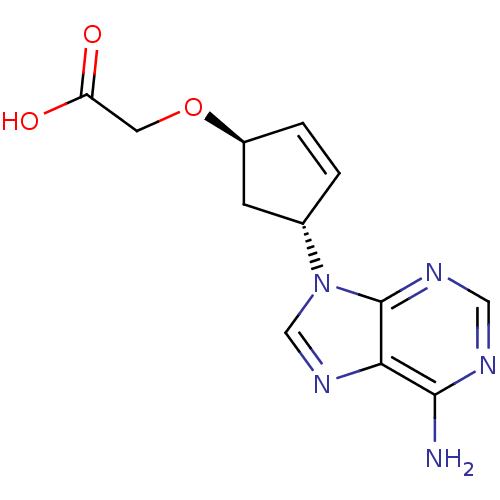
(2-{[(1R,4R)-4-(6-amino-9H-purin-9-yl)cyclopent-2-e...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1C[C@@H](OCC(O)=O)C=C1 |c:20| Show InChI InChI=1S/C12H13N5O3/c13-11-10-12(15-5-14-11)17(6-16-10)7-1-2-8(3-7)20-4-9(18)19/h1-2,5-8H,3-4H2,(H,18,19)(H2,13,14,15)/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 5
(Homo sapiens (Human)) | BDBM50119852

(2-{[(1S,3S)-3-(6-amino-9H-purin-9-yl)cyclopentyl]o...)Show InChI InChI=1S/C12H15N5O3/c13-11-10-12(15-5-14-11)17(6-16-10)7-1-2-8(3-7)20-4-9(18)19/h5-8H,1-4H2,(H,18,19)(H2,13,14,15)/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Type V Adenyl Cyclase enzyme |
Bioorg Med Chem Lett 12: 3089-92 (2002)
BindingDB Entry DOI: 10.7270/Q2T43SFX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data