

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290192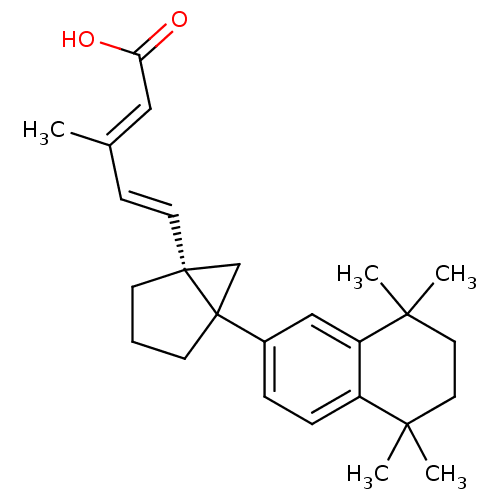 (3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032671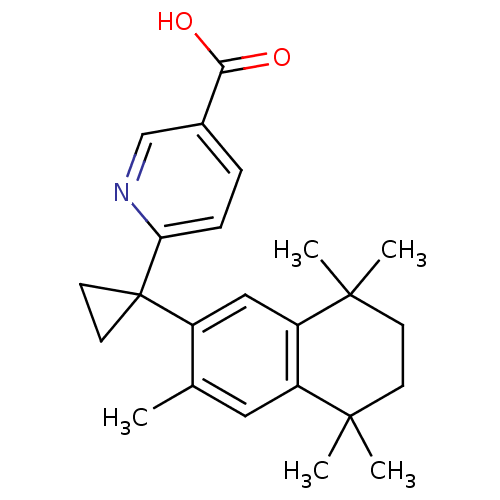 (6-[1-(3,5,5,8,8-PENTAMETHYL-5,6,7,8-TETRAHYDRONAPH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032666 (6-[1-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM31883 (9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR gamma | J Med Chem 41: 1679-87 (1998) Article DOI: 10.1021/jm970635h BindingDB Entry DOI: 10.7270/Q20864F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290187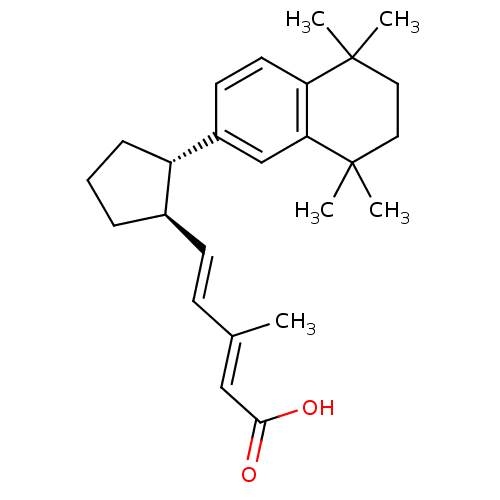 ((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50122351 ((2E,4E)-3-Methyl-6-[1-(5,5,8,8-tetramethyl-5,6,7,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expresing mouse Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 13: 261-4 (2002) BindingDB Entry DOI: 10.7270/Q29P310V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290186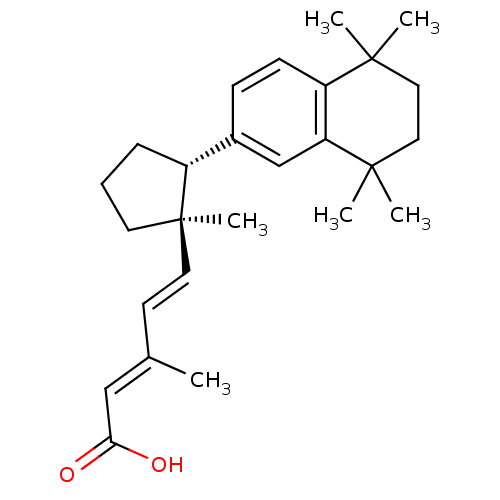 ((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032664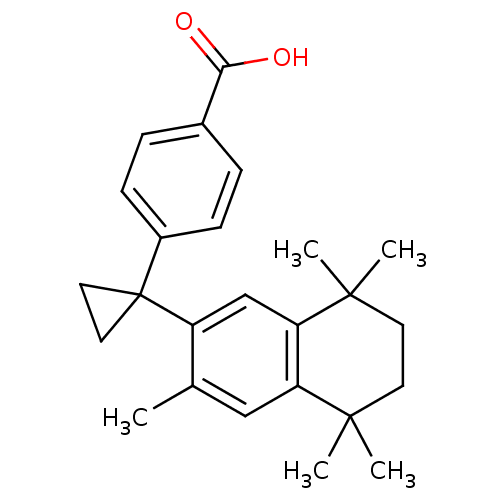 (4-[1-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-ATRA binding to retinoic acid receptor RAR beta | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50122349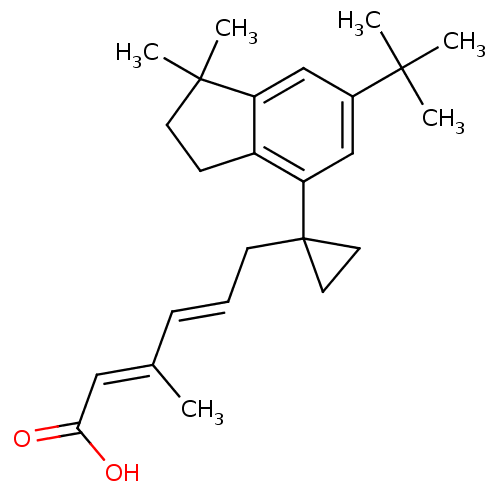 ((2E,4E)-6-[1-(6-tert-Butyl-1,1-dimethyl-indan-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expresing mouse Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 13: 261-4 (2002) BindingDB Entry DOI: 10.7270/Q29P310V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50080526 (1,4,4-Trimethyl-1,2,3,4-tetrahydro-quinoline-6-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
University of Oklahoma Health Sciences Center Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing Retinoid X receptor RXR gamma | J Med Chem 42: 3602-14 (1999) Article DOI: 10.1021/jm9900974 BindingDB Entry DOI: 10.7270/Q2J38RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50122348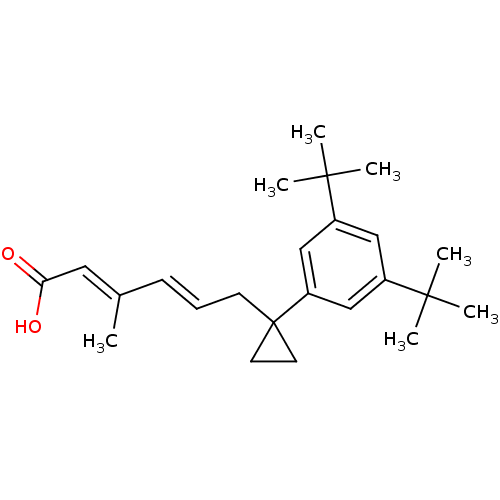 ((2E,4E)-6-[1-(3,5-Di-tert-butyl-phenyl)-cyclopropy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expresing mouse Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 13: 261-4 (2002) BindingDB Entry DOI: 10.7270/Q29P310V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50122350 ((2E,4E)-3-Methyl-6-[1-(5,5,8,8-tetramethyl-5,6,7,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expresing mouse Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 13: 261-4 (2002) BindingDB Entry DOI: 10.7270/Q29P310V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290194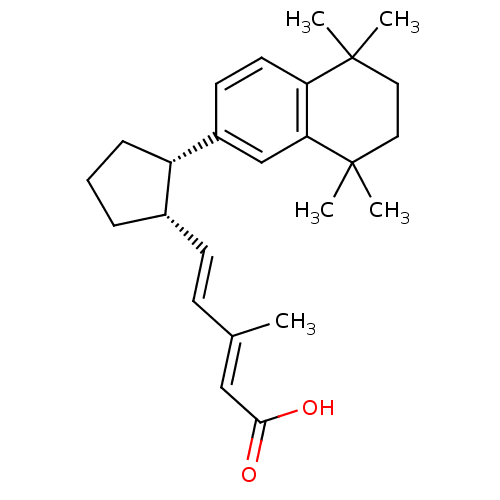 ((2E,4E)-3-Methyl-5-[(1S,2S)-2-(5,5,8,8-tetramethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR beta | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50080524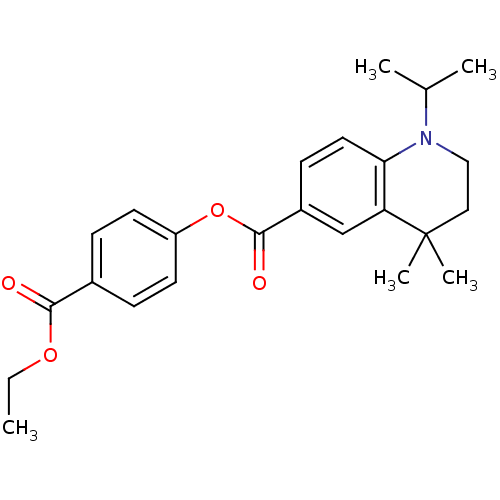 (1-Isopropyl-4,4-dimethyl-1,2,3,4-tetrahydro-quinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
University of Oklahoma Health Sciences Center Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing Retinoid X receptor RXR gamma | J Med Chem 42: 3602-14 (1999) Article DOI: 10.1021/jm9900974 BindingDB Entry DOI: 10.7270/Q2J38RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50122352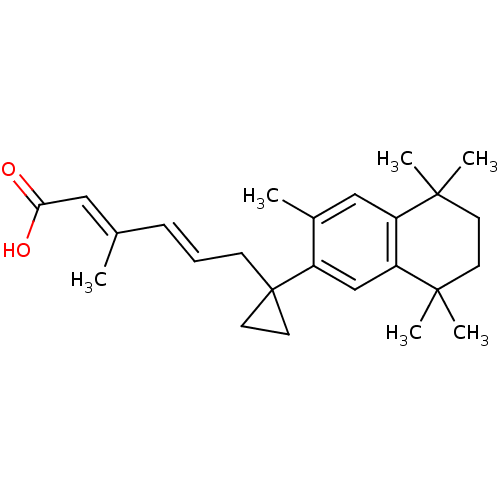 ((2E,4E)-3-Methyl-6-[1-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expresing mouse Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 13: 261-4 (2002) BindingDB Entry DOI: 10.7270/Q29P310V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040016 (4-[1-(3-Chloro-5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290191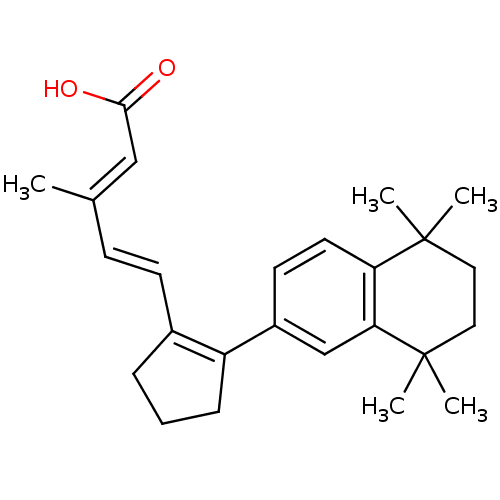 (3-Methyl-5-[2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50082041 (4-iminomethylphenyl 4,4-dimethyl-6-chromanecarboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
University of Oklahoma Health Sciences Center Curated by ChEMBL | Assay Description Transcriptional activation of CV-1 cells expressing murine Retinoid X receptor RXR gamma | J Med Chem 42: 4434-45 (1999) BindingDB Entry DOI: 10.7270/Q2891530 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50064252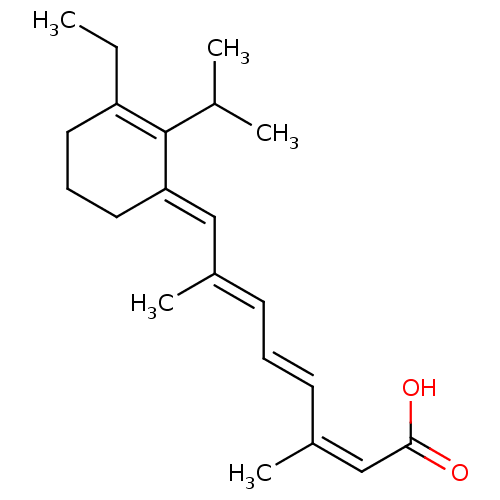 ((2Z,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR gamma | J Med Chem 41: 1679-87 (1998) Article DOI: 10.1021/jm970635h BindingDB Entry DOI: 10.7270/Q20864F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040012 (4-[1-(3-Bromo-5,5,8,8-tetramethyl-5,6,7,8-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290188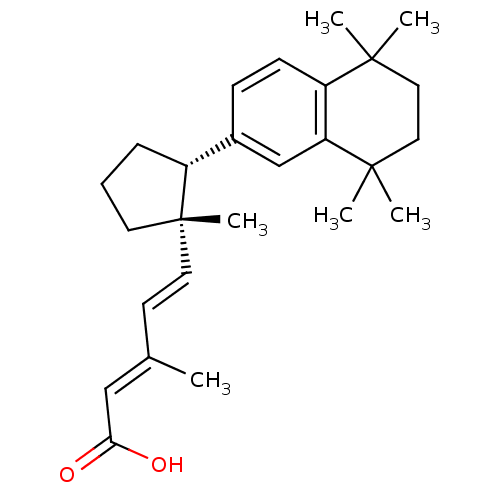 ((2E,4E)-3-Methyl-5-[(1S,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032674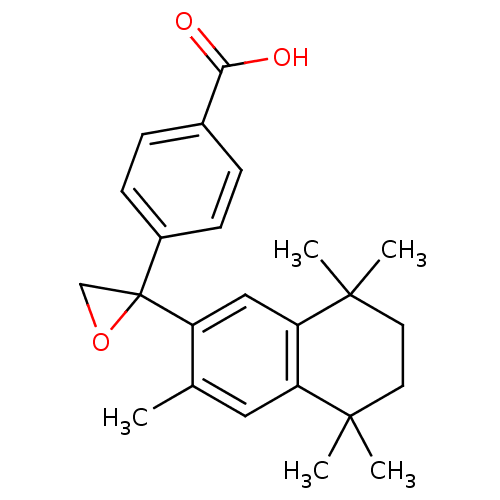 (4-[2-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 47 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50080525 (1,4,4,7-Tetramethyl-1,2,3,4-tetrahydro-quinoline-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
University of Oklahoma Health Sciences Center Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing Retinoid X receptor RXR gamma | J Med Chem 42: 3602-14 (1999) Article DOI: 10.1021/jm9900974 BindingDB Entry DOI: 10.7270/Q2J38RRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM31892 (9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | n/a | n/a | 124 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expresing mouse Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 13: 261-4 (2002) BindingDB Entry DOI: 10.7270/Q29P310V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290193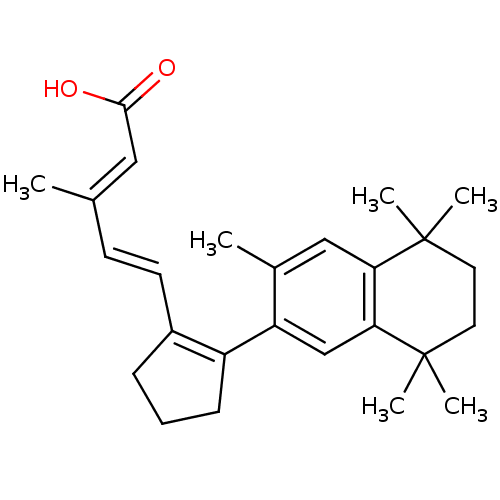 (3-Methyl-5-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 128 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040017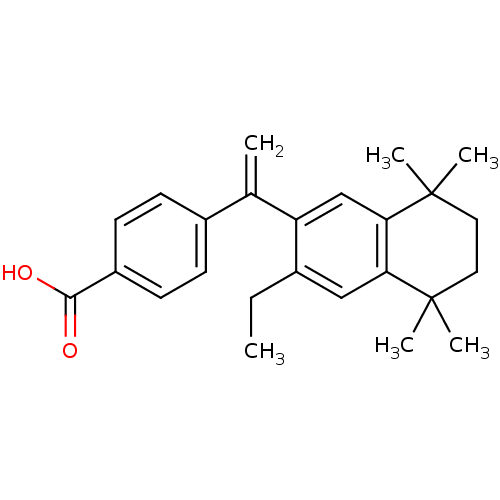 (4-[1-(3-Ethyl-5,5,8,8-tetramethyl-5,6,7,8-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 136 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290189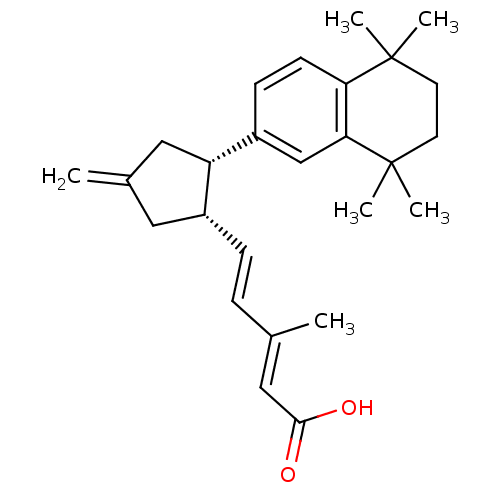 ((2E,4E)-3-Methyl-5-[(1S,2S)-4-methylene-2-(5,5,8,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 204 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290190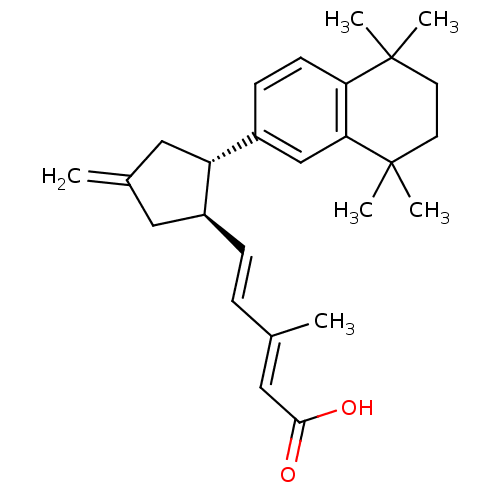 ((2E,4E)-3-Methyl-5-[(1R,2S)-4-methylene-2-(5,5,8,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 212 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040013 (4-(3-Chloro-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 225 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040021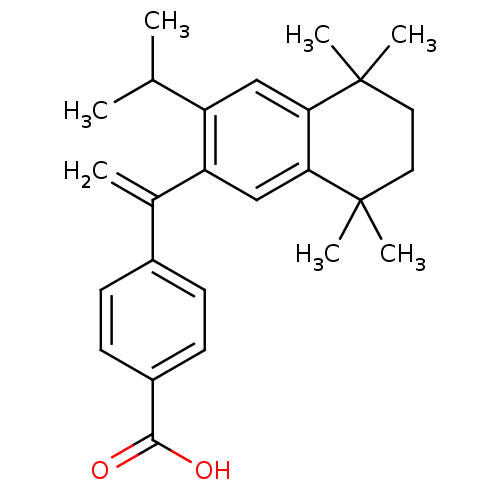 (4-[1-(3-Isopropyl-5,5,8,8-tetramethyl-5,6,7,8-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 227 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040015 (4-[1-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,8-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032668 (4-[2-Methyl-1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032667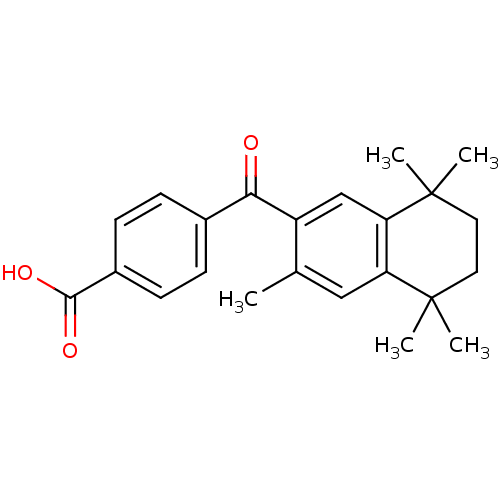 (4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 246 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032667 (4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 246 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040011 (4-(3-Bromo-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 261 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50082042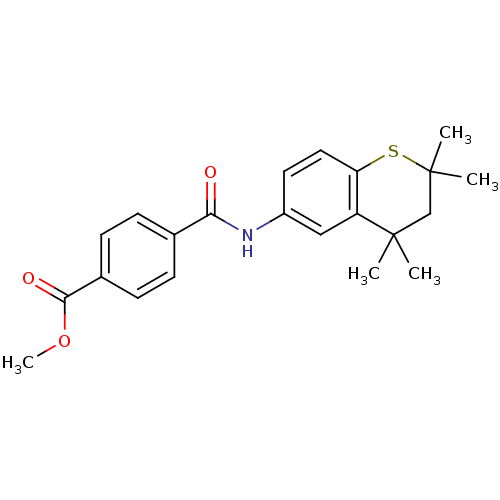 (CHEMBL139536 | N-(2,2,4,4-Tetramethyl-thiochroman-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 302 | n/a | n/a | n/a | n/a |
University of Oklahoma Health Sciences Center Curated by ChEMBL | Assay Description Transcriptional activation of CV-1 cells expressing murine Retinoid X receptor RXR gamma | J Med Chem 42: 4434-45 (1999) BindingDB Entry DOI: 10.7270/Q2891530 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032677 (4-[1-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-nap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040018 (4-(3-Isopropyl-5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 357 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040014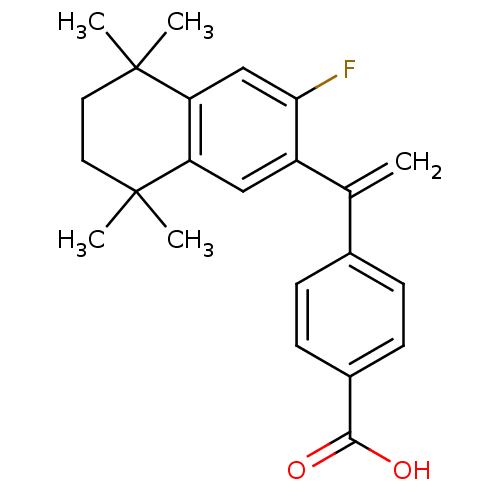 (4-[1-(3-Fluoro-5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 383 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040010 (4-(3-Ethyl-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 384 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50033079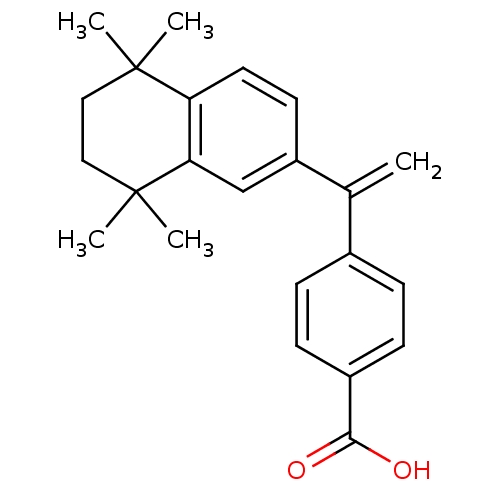 (4-[1-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 404 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032676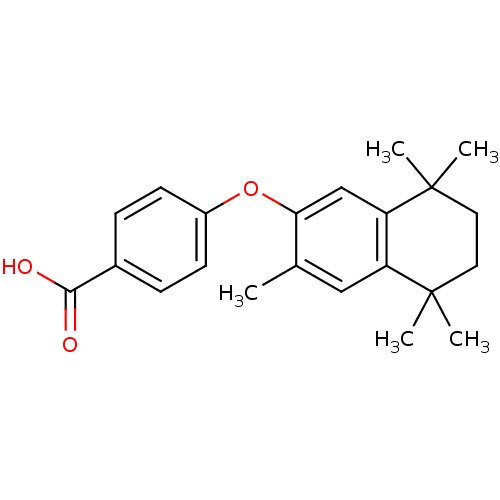 (4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032663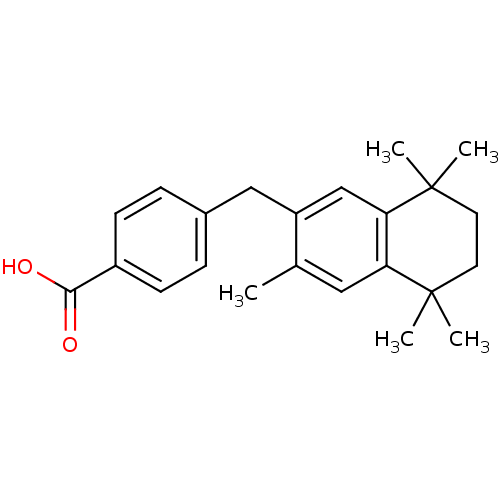 (4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032662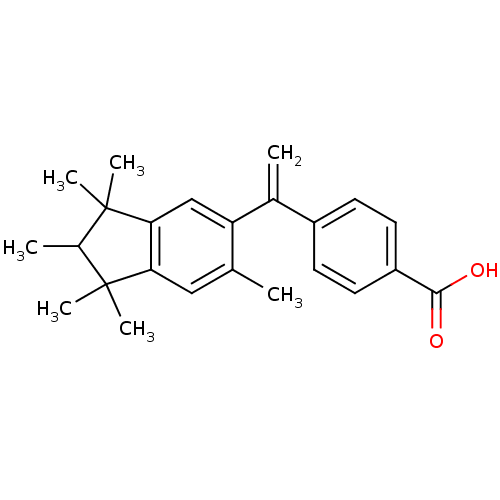 (4-[1-(1,1,2,3,3,6-Hexamethyl-indan-5-yl)-vinyl]-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50040024 (4-[1-(3-Hydroxy-5,5,8,8-tetramethyl-5,6,7,8-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 558 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration against retinoid receptor isoform (RXR gamma) expressed in CV-1 cells | J Med Chem 37: 2930-41 (1994) BindingDB Entry DOI: 10.7270/Q27080G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032665 (4-[1-(3,5,5-Trimethyl-5,6,7,8-tetrahydro-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50032670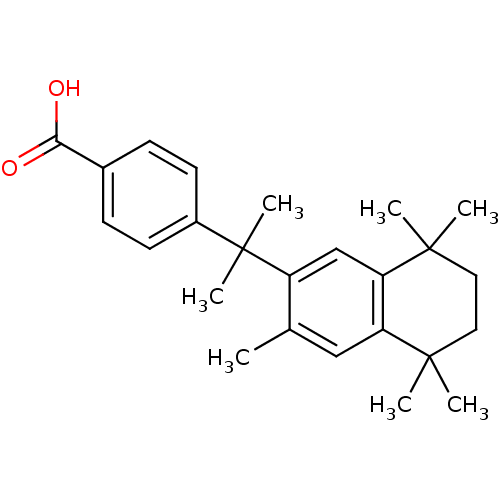 (4-[1-Methyl-1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 650 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Ability to activate gene expression at Retinoic acid receptor RXR-gamma was evaluated in a cotransfection assay. | J Med Chem 38: 3146-55 (1995) BindingDB Entry DOI: 10.7270/Q2542MMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |