 Found 49 hits of ic50 data for polymerid = 50002574
Found 49 hits of ic50 data for polymerid = 50002574 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014986
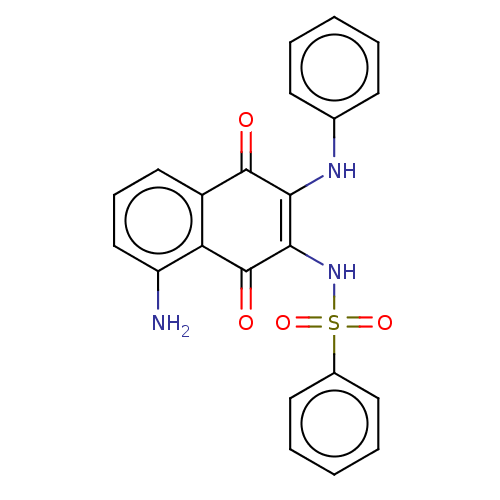
(CHEMBL3262086)Show SMILES Nc1cccc2C(=O)C(Nc3ccccc3)=C(NS(=O)(=O)c3ccccc3)C(=O)c12 |t:16| Show InChI InChI=1S/C22H17N3O4S/c23-17-13-7-12-16-18(17)22(27)20(25-30(28,29)15-10-5-2-6-11-15)19(21(16)26)24-14-8-3-1-4-9-14/h1-13,24-25H,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50275074

((Z)-5-(3',5'-Diiodo-4'-hydroxybenzylidene)-2-thiox...)Show SMILES Oc1c(I)cc(C=C2SC(S)=NC2=O)cc1I |w:6.5,c:10| Show InChI InChI=1S/C10H5I2NO2S2/c11-5-1-4(2-6(12)8(5)14)3-7-9(15)13-10(16)17-7/h1-3,14H,(H,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of NAT1 in human ZR75 cell lysate assessed as acetylation of aryl amine using PABA as substrate |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50275074

((Z)-5-(3',5'-Diiodo-4'-hydroxybenzylidene)-2-thiox...)Show SMILES Oc1c(I)cc(C=C2SC(S)=NC2=O)cc1I |w:6.5,c:10| Show InChI InChI=1S/C10H5I2NO2S2/c11-5-1-4(2-6(12)8(5)14)3-7-9(15)13-10(16)17-7/h1-3,14H,(H,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014944
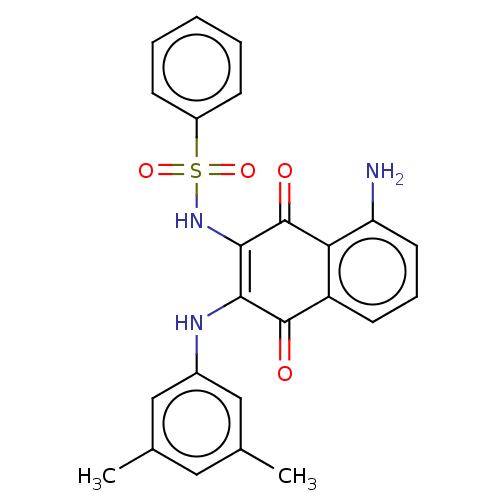
(CHEMBL3262085)Show SMILES Cc1cc(C)cc(NC2=C(NS(=O)(=O)c3ccccc3)C(=O)c3c(N)cccc3C2=O)c1 |c:8| Show InChI InChI=1S/C24H21N3O4S/c1-14-11-15(2)13-16(12-14)26-21-22(27-32(30,31)17-7-4-3-5-8-17)24(29)20-18(23(21)28)9-6-10-19(20)25/h3-13,26-27H,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50274853
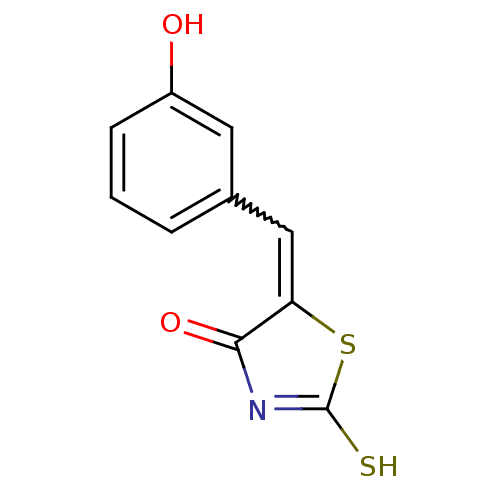
((Z)-5-(3'-Hydroxybenzylidene)-2-thioxothiazolidin-...)Show InChI InChI=1S/C10H7NO2S2/c12-7-3-1-2-6(4-7)5-8-9(13)11-10(14)15-8/h1-5,12H,(H,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014914

(CHEMBL3262049)Show SMILES Brc1ccc(NC2=C(NS(=O)(=O)c3ccccc3)C(=O)c3ccccc3C2=O)cc1 |c:6| Show InChI InChI=1S/C22H15BrN2O4S/c23-14-10-12-15(13-11-14)24-19-20(25-30(28,29)16-6-2-1-3-7-16)22(27)18-9-5-4-8-17(18)21(19)26/h1-13,24-25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50368688

(CHEMBL2047717)Show InChI InChI=1S/C10H7NO3S/c12-7-4-2-1-3-6(7)5-8-9(13)11-10(14)15-8/h1-5,12H,(H,11,13,14)/b8-5- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) arylamine N-acetyltransferase 1 |
Citation and Details
Article DOI: 10.1007/s00044-012-0057-3
BindingDB Entry DOI: 10.7270/Q2X351BT |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50092273

((Z)-5-(2'-Hydroxybenzylidene)-2-thioxothiazolidin-...)Show InChI InChI=1S/C10H7NO2S2/c12-7-4-2-1-3-6(7)5-8-9(13)11-10(14)15-8/h1-5,12H,(H,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50274890

((Z)-3-Amino-5-(4'-hydroxy-3',5-diiodobenzylidene)-...)Show InChI InChI=1S/C10H6I2N2O2S2/c11-5-1-4(2-6(12)8(5)15)3-7-9(16)14(13)10(17)18-7/h1-3,15H,13H2/b7-3- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014912
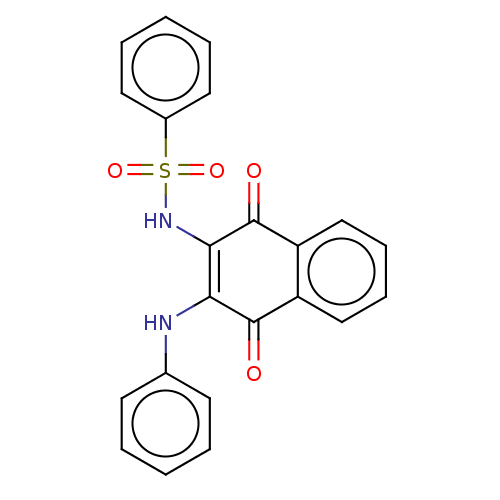
(CHEMBL1568345)Show SMILES O=C1C(Nc2ccccc2)=C(NS(=O)(=O)c2ccccc2)C(=O)c2ccccc12 |t:10| Show InChI InChI=1S/C22H16N2O4S/c25-21-17-13-7-8-14-18(17)22(26)20(19(21)23-15-9-3-1-4-10-15)24-29(27,28)16-11-5-2-6-12-16/h1-14,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014924

(CHEMBL3262055)Show SMILES Brc1ccc(OC2=C(NS(=O)(=O)c3ccccc3)C(=O)c3ccccc3C2=O)cc1 |c:6| Show InChI InChI=1S/C22H14BrNO5S/c23-14-10-12-15(13-11-14)29-22-19(24-30(27,28)16-6-2-1-3-7-16)20(25)17-8-4-5-9-18(17)21(22)26/h1-13,24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014943

(CHEMBL3262084)Show SMILES Cc1cc(C)cc(NC2=C(NS(=O)(=O)c3ccccc3)C(=O)c3cc(N)ccc3C2=O)c1 |c:8| Show InChI InChI=1S/C24H21N3O4S/c1-14-10-15(2)12-17(11-14)26-21-22(27-32(30,31)18-6-4-3-5-7-18)24(29)20-13-16(25)8-9-19(20)23(21)28/h3-13,26-27H,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014922

(CHEMBL3262053)Show SMILES O=C1C(NS(=O)(=O)c2ccccc2)=C(Oc2ccccc2)C(=O)c2ccccc12 |t:13| Show InChI InChI=1S/C22H15NO5S/c24-20-17-13-7-8-14-18(17)21(25)22(28-15-9-3-1-4-10-15)19(20)23-29(26,27)16-11-5-2-6-12-16/h1-14,23H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50274887
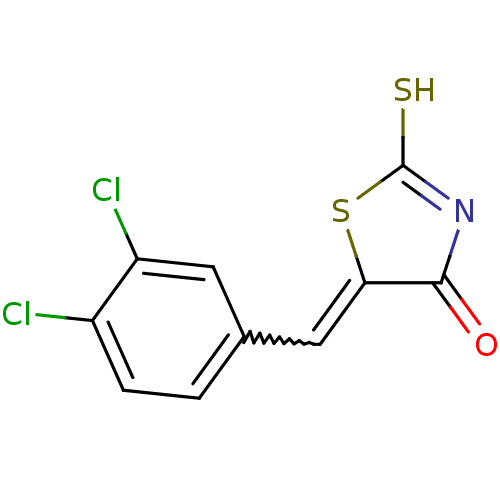
((Z)-5-(3',4'-Dichlorobenzylidene)-2-thioxothiazoli...)Show SMILES SC1=NC(=O)C(S1)=Cc1ccc(Cl)c(Cl)c1 |w:7.8,t:1| Show InChI InChI=1S/C10H5Cl2NOS2/c11-6-2-1-5(3-7(6)12)4-8-9(14)13-10(15)16-8/h1-4H,(H,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50240730
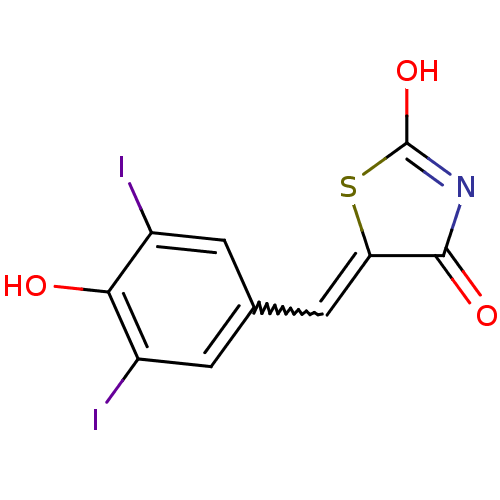
((Z)-5-(4'-Hydroxy-3',5'-diiodobenzylidene)thiazoli...)Show SMILES OC1=NC(=O)C(S1)=Cc1cc(I)c(O)c(I)c1 |w:7.8,t:1| Show InChI InChI=1S/C10H5I2NO3S/c11-5-1-4(2-6(12)8(5)14)3-7-9(15)13-10(16)17-7/h1-3,14H,(H,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50274886

((Z)-5-(2'-Methylbenzylidene)-2-thioxothiazolidin-4...)Show InChI InChI=1S/C11H9NOS2/c1-7-4-2-3-5-8(7)6-9-10(13)12-11(14)15-9/h2-6H,1H3,(H,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014913

(CHEMBL3262048)Show SMILES Cc1cc(C)cc(NC2=C(NS(=O)(=O)c3ccccc3)C(=O)c3ccccc3C2=O)c1 |c:8| Show InChI InChI=1S/C24H20N2O4S/c1-15-12-16(2)14-17(13-15)25-21-22(26-31(29,30)18-8-4-3-5-9-18)24(28)20-11-7-6-10-19(20)23(21)27/h3-14,25-26H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014942
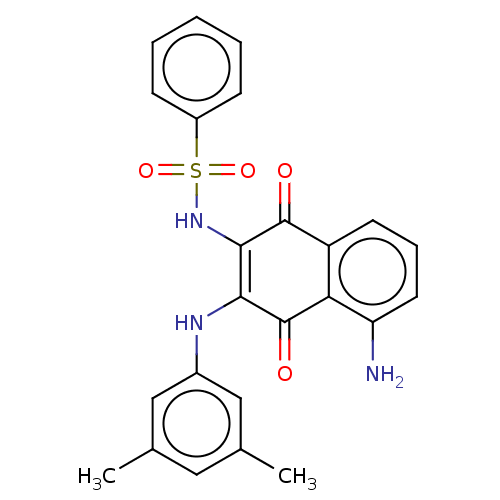
(CHEMBL3262082)Show SMILES Cc1cc(C)cc(NC2=C(NS(=O)(=O)c3ccccc3)C(=O)c3cccc(N)c3C2=O)c1 |c:8| Show InChI InChI=1S/C24H21N3O4S/c1-14-11-15(2)13-16(12-14)26-21-22(27-32(30,31)17-7-4-3-5-8-17)23(28)18-9-6-10-19(25)20(18)24(21)29/h3-13,26-27H,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50181730
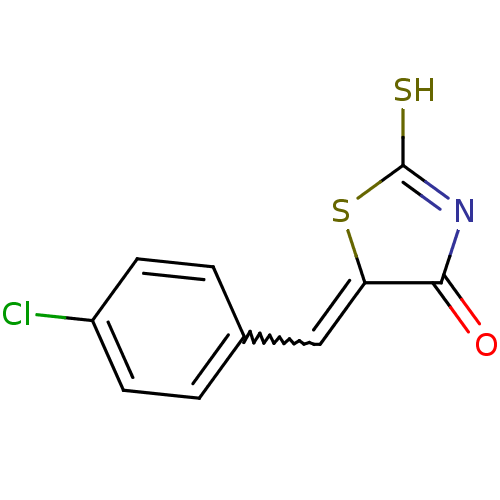
((Z)-5-(4'-Chlorobenzylidene)-2-thioxothiazolidin-4...)Show InChI InChI=1S/C10H6ClNOS2/c11-7-3-1-6(2-4-7)5-8-9(13)12-10(14)15-8/h1-5H,(H,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014987
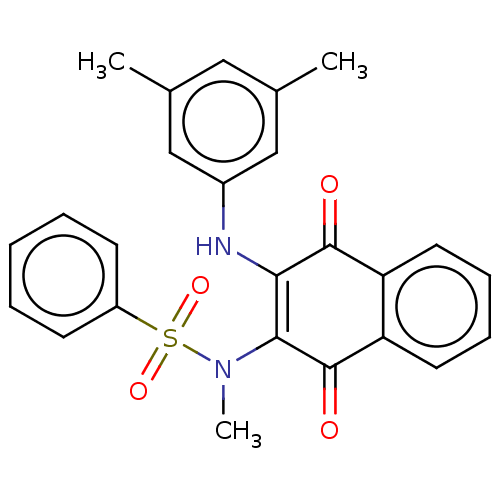
(CHEMBL3262087)Show SMILES CN(C1=C(Nc2cc(C)cc(C)c2)C(=O)c2ccccc2C1=O)S(=O)(=O)c1ccccc1 |c:2| Show InChI InChI=1S/C25H22N2O4S/c1-16-13-17(2)15-18(14-16)26-22-23(25(29)21-12-8-7-11-20(21)24(22)28)27(3)32(30,31)19-9-5-4-6-10-19/h4-15,26H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014938

(CHEMBL3262077)Show SMILES Cc1cc(C)cc(NC2=C(NS(=O)(=O)c3ccccc3)C(=O)c3cccc(c3C2=O)[N+]([O-])=O)c1 |c:8| Show InChI InChI=1S/C24H19N3O6S/c1-14-11-15(2)13-16(12-14)25-21-22(26-34(32,33)17-7-4-3-5-8-17)23(28)18-9-6-10-19(27(30)31)20(18)24(21)29/h3-13,25-26H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50274889
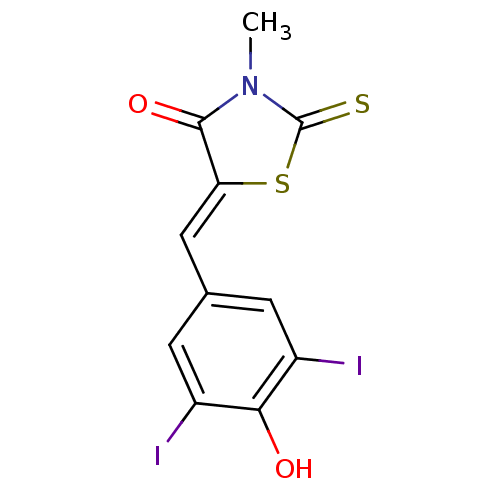
((Z)-5-(4'-Hydroxy-3',5'-diiodobenzylidene)-3-methy...)Show InChI InChI=1S/C11H7I2NO2S2/c1-14-10(16)8(18-11(14)17)4-5-2-6(12)9(15)7(13)3-5/h2-4,15H,1H3/b8-4- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014925
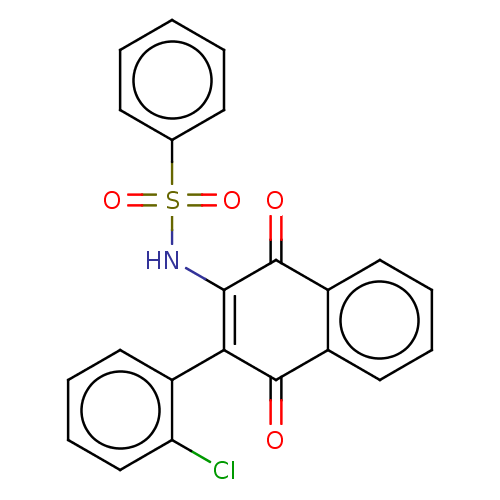
(CHEMBL3262056)Show SMILES Clc1ccccc1C1=C(NS(=O)(=O)c2ccccc2)C(=O)c2ccccc2C1=O |c:8| Show InChI InChI=1S/C22H14ClNO4S/c23-18-13-7-6-12-17(18)19-20(24-29(27,28)14-8-2-1-3-9-14)22(26)16-11-5-4-10-15(16)21(19)25/h1-13,24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50274888

((Z)-5-(4'-Biphenylmethylene)-2-thioxothiazolidin-4...)Show SMILES SC1=NC(=O)C(S1)=Cc1ccc(cc1)-c1ccccc1 |w:7.8,t:1| Show InChI InChI=1S/C16H11NOS2/c18-15-14(20-16(19)17-15)10-11-6-8-13(9-7-11)12-4-2-1-3-5-12/h1-10H,(H,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014928

(CHEMBL3262059)Show SMILES O=Cc1cccc(c1)C1=C(NS(=O)(=O)c2ccccc2)C(=O)c2ccccc2C1=O |c:9| Show InChI InChI=1S/C23H15NO5S/c25-14-15-7-6-8-16(13-15)20-21(24-30(28,29)17-9-2-1-3-10-17)23(27)19-12-5-4-11-18(19)22(20)26/h1-14,24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014930
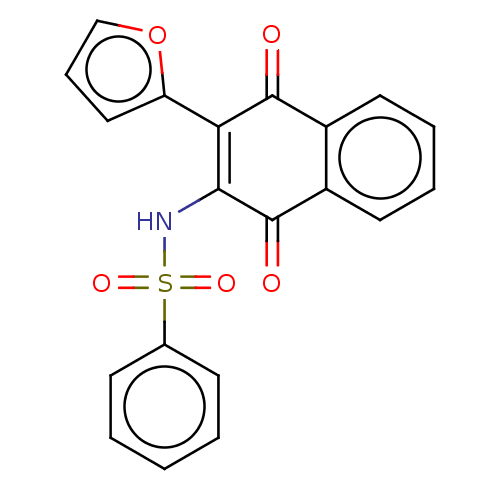
(CHEMBL3262061)Show SMILES O=C1C(NS(=O)(=O)c2ccccc2)=C(c2ccco2)C(=O)c2ccccc12 |t:13| Show InChI InChI=1S/C20H13NO5S/c22-19-14-9-4-5-10-15(14)20(23)18(17(19)16-11-6-12-26-16)21-27(24,25)13-7-2-1-3-8-13/h1-12,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014932

(CHEMBL3262073)Show SMILES O=Cc1cccc(c1)C1=C(NC(=O)c2ccccc2)C(=O)c2ccccc2C1=O |c:9| Show InChI InChI=1S/C24H15NO4/c26-14-15-7-6-10-17(13-15)20-21(25-24(29)16-8-2-1-3-9-16)23(28)19-12-5-4-11-18(19)22(20)27/h1-14H,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014931
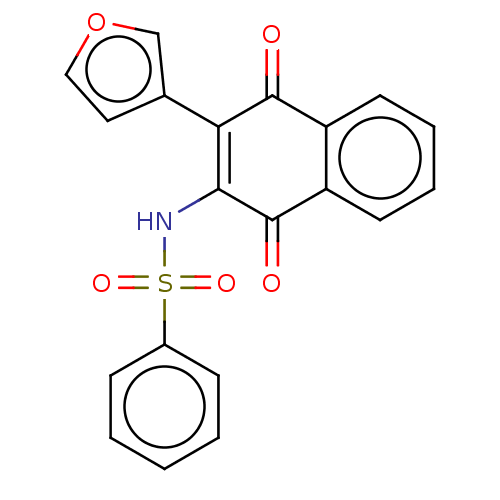
(CHEMBL3262062)Show SMILES O=C1C(NS(=O)(=O)c2ccccc2)=C(c2ccoc2)C(=O)c2ccccc12 |t:13| Show InChI InChI=1S/C20H13NO5S/c22-19-15-8-4-5-9-16(15)20(23)18(17(19)13-10-11-26-12-13)21-27(24,25)14-6-2-1-3-7-14/h1-12,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014929

(CHEMBL3262060)Show SMILES O=Cc1ccc(cc1)C1=C(NS(=O)(=O)c2ccccc2)C(=O)c2ccccc2C1=O |c:9| Show InChI InChI=1S/C23H15NO5S/c25-14-15-10-12-16(13-11-15)20-21(24-30(28,29)17-6-2-1-3-7-17)23(27)19-9-5-4-8-18(19)22(20)26/h1-14,24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014923

(CHEMBL3262054)Show SMILES Cc1cc(C)cc(OC2=C(NS(=O)(=O)c3ccccc3)C(=O)c3ccccc3C2=O)c1 |c:8| Show InChI InChI=1S/C24H19NO5S/c1-15-12-16(2)14-17(13-15)30-24-21(25-31(28,29)18-8-4-3-5-9-18)22(26)19-10-6-7-11-20(19)23(24)27/h3-14,25H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014927
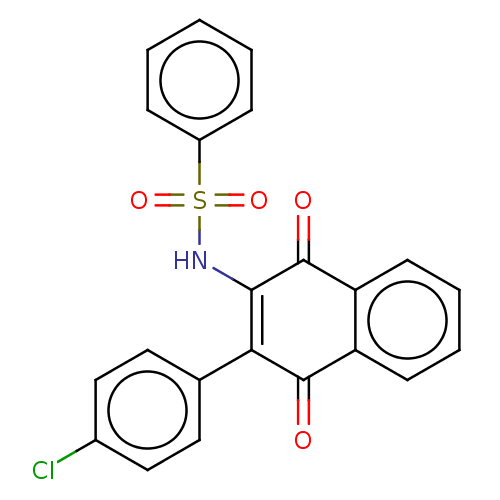
(CHEMBL3262058)Show SMILES Clc1ccc(cc1)C1=C(NS(=O)(=O)c2ccccc2)C(=O)c2ccccc2C1=O |c:8| Show InChI InChI=1S/C22H14ClNO4S/c23-15-12-10-14(11-13-15)19-20(24-29(27,28)16-6-2-1-3-7-16)22(26)18-9-5-4-8-17(18)21(19)25/h1-13,24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50241154
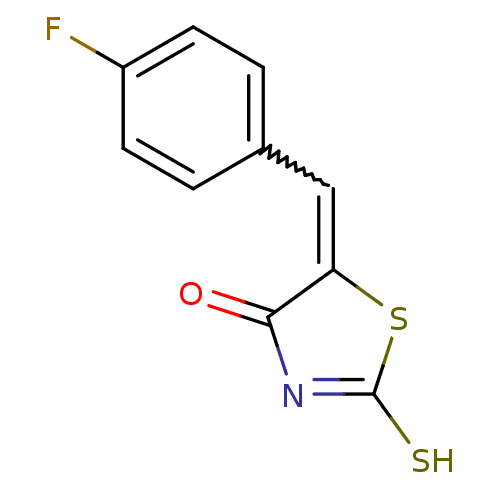
((Z)-5-(4'-Fluorobenzylidene)-2-thioxothiazolidin-4...)Show InChI InChI=1S/C10H6FNOS2/c11-7-3-1-6(2-4-7)5-8-9(13)12-10(14)15-8/h1-5H,(H,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50092271

((Z)-5-Benzylidene-2-thioxothiazolidin-4-one | (Z)-...)Show InChI InChI=1S/C10H7NOS2/c12-9-8(14-10(13)11-9)6-7-4-2-1-3-5-7/h1-6H,(H,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014926

(CHEMBL3262057)Show SMILES Clc1cccc(c1)C1=C(NS(=O)(=O)c2ccccc2)C(=O)c2ccccc2C1=O |c:8| Show InChI InChI=1S/C22H14ClNO4S/c23-15-8-6-7-14(13-15)19-20(24-29(27,28)16-9-2-1-3-10-16)22(26)18-12-5-4-11-17(18)21(19)25/h1-13,24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50247599
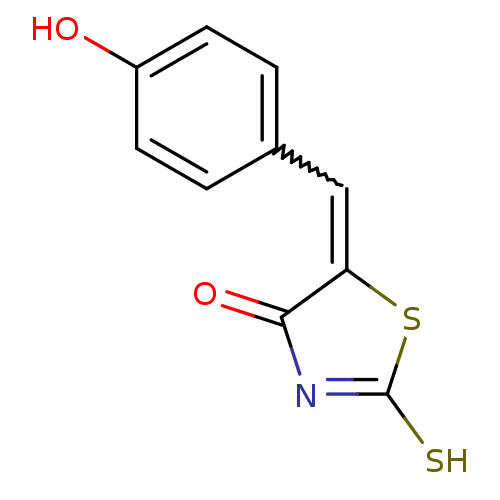
((Z)-5-(4'-Hydroxybenzylidene)-2-thioxothiazolidin-...)Show InChI InChI=1S/C10H7NO2S2/c12-7-3-1-6(2-4-7)5-8-9(13)11-10(14)15-8/h1-5,12H,(H,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50274855

((Z)-5-(3'-Methylbenzylidene)-2-thioxothiazolidin-4...)Show InChI InChI=1S/C11H9NOS2/c1-7-3-2-4-8(5-7)6-9-10(13)12-11(14)15-9/h2-6H,1H3,(H,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50274854

((Z)-5-(2'-Methoxybenzylidene)-2-thioxothiazolidin-...)Show InChI InChI=1S/C11H9NO2S2/c1-14-8-5-3-2-4-7(8)6-9-10(13)12-11(15)16-9/h2-6H,1H3,(H,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014933

(CHEMBL3262074)Show SMILES O=C(NC1=C(c2ccoc2)C(=O)c2ccccc2C1=O)c1ccccc1 |c:3| Show InChI InChI=1S/C21H13NO4/c23-19-15-8-4-5-9-16(15)20(24)18(17(19)14-10-11-26-12-14)22-21(25)13-6-2-1-3-7-13/h1-12H,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014937

(CHEMBL3262076)Show SMILES O=C(Cc1ccccc1)NC1=C(c2ccoc2)C(=O)c2ccccc2C1=O |c:11| Show InChI InChI=1S/C22H15NO4/c24-18(12-14-6-2-1-3-7-14)23-20-19(15-10-11-27-13-15)21(25)16-8-4-5-9-17(16)22(20)26/h1-11,13H,12H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014934
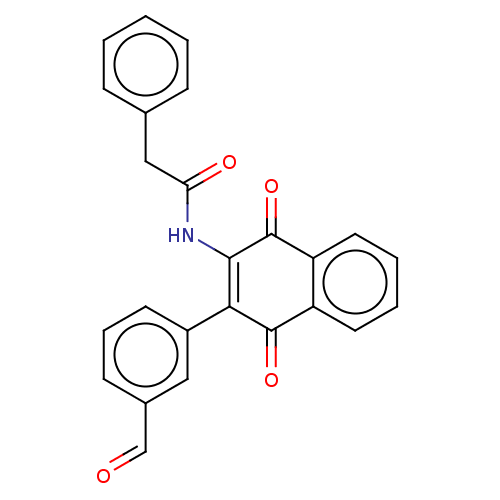
(CHEMBL3262075)Show SMILES O=Cc1cccc(c1)C1=C(NC(=O)Cc2ccccc2)C(=O)c2ccccc2C1=O |c:9| Show InChI InChI=1S/C25H17NO4/c27-15-17-9-6-10-18(13-17)22-23(26-21(28)14-16-7-2-1-3-8-16)25(30)20-12-5-4-11-19(20)24(22)29/h1-13,15H,14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014916
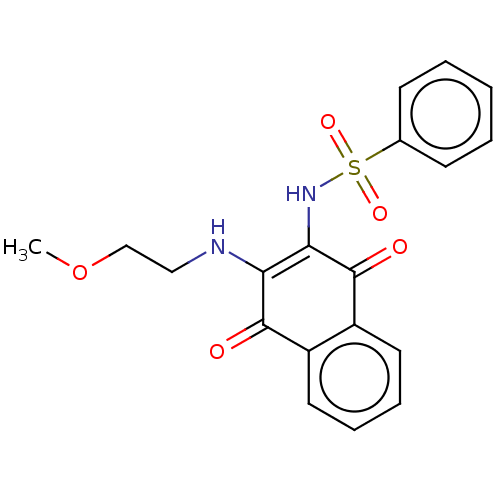
(CHEMBL3262050)Show SMILES COCCNC1=C(NS(=O)(=O)c2ccccc2)C(=O)c2ccccc2C1=O |c:5| Show InChI InChI=1S/C19H18N2O5S/c1-26-12-11-20-16-17(21-27(24,25)13-7-3-2-4-8-13)19(23)15-10-6-5-9-14(15)18(16)22/h2-10,20-21H,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014920

(CHEMBL3262051)Show SMILES O=C1C(NC2CCCC2)=C(NS(=O)(=O)c2ccccc2)C(=O)c2ccccc12 |t:9| Show InChI InChI=1S/C21H20N2O4S/c24-20-16-12-6-7-13-17(16)21(25)19(18(20)22-14-8-4-5-9-14)23-28(26,27)15-10-2-1-3-11-15/h1-3,6-7,10-14,22-23H,4-5,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014921

(CHEMBL3262052)Show SMILES O=C1C(NCc2ccccc2)=C(NS(=O)(=O)c2ccccc2)C(=O)c2ccccc12 |t:11| Show InChI InChI=1S/C23H18N2O4S/c26-22-18-13-7-8-14-19(18)23(27)21(20(22)24-15-16-9-3-1-4-10-16)25-30(28,29)17-11-5-2-6-12-17/h1-14,24-25H,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50241156
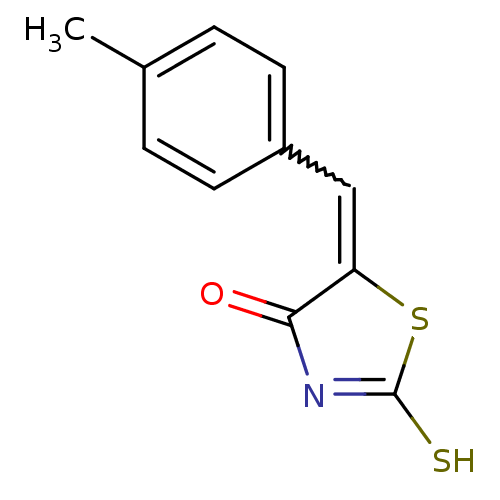
((Z)-5-(4'-Methylbenzylidene)-2-thioxothiazolidin-4...)Show InChI InChI=1S/C11H9NOS2/c1-7-2-4-8(5-3-7)6-9-10(13)12-11(14)15-9/h2-6H,1H3,(H,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50241489

((Z)-5-(3'-Methoxybenzylidene)-2-thioxothiazolidin-...)Show InChI InChI=1S/C11H9NO2S2/c1-14-8-4-2-3-7(5-8)6-9-10(13)12-11(15)16-9/h2-6H,1H3,(H,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014941

(CHEMBL3262081)Show SMILES [O-][N+](=O)c1cccc2C(=O)C(Nc3ccccc3)=C(NS(=O)(=O)c3ccccc3)C(=O)c12 |t:18| Show InChI InChI=1S/C22H15N3O6S/c26-21-16-12-7-13-17(25(28)29)18(16)22(27)20(19(21)23-14-8-3-1-4-9-14)24-32(30,31)15-10-5-2-6-11-15/h1-13,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014940
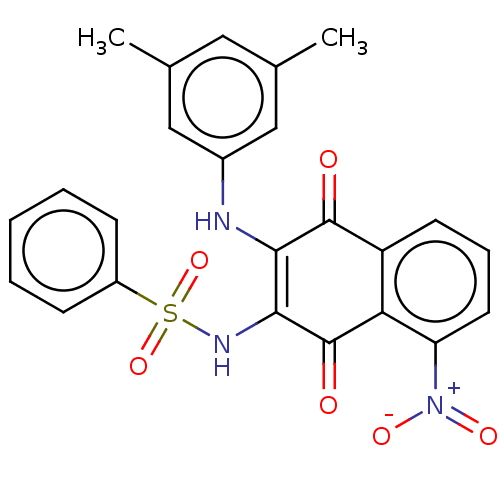
(CHEMBL3262080)Show SMILES Cc1cc(C)cc(NC2=C(NS(=O)(=O)c3ccccc3)C(=O)c3c(cccc3[N+]([O-])=O)C2=O)c1 |c:8| Show InChI InChI=1S/C24H19N3O6S/c1-14-11-15(2)13-16(12-14)25-21-22(26-34(32,33)17-7-4-3-5-8-17)24(29)20-18(23(21)28)9-6-10-19(20)27(30)31/h3-13,25-26H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50014939
(CHEMBL3262078)Show SMILES Cc1cc(C)cc(NC2=C(NS(=O)(=O)c3ccccc3)C(=O)c3ccc(cc3C2=O)[N+]([O-])=O)c1 |c:8| Show InChI InChI=1S/C24H19N3O6S/c1-14-10-15(2)12-16(11-14)25-21-22(26-34(32,33)18-6-4-3-5-7-18)23(28)19-9-8-17(27(30)31)13-20(19)24(21)29/h3-13,25-26H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human NAT1 assessed as rate of free thiol coenzyme A production using arylamine and AcCoA as substrate by Ellman's method |
Bioorg Med Chem 22: 3030-54 (2014)
Article DOI: 10.1016/j.bmc.2014.03.015
BindingDB Entry DOI: 10.7270/Q2S75HVG |
More data for this
Ligand-Target Pair | |
Arylamine N-acetyltransferase 1
(Homo sapiens (Human)) | BDBM50241157

((Z)-5-(4'-Methoxybenzylidene)-2-thioxothiazolidin-...)Show InChI InChI=1S/C11H9NO2S2/c1-14-8-4-2-7(3-5-8)6-9-10(13)12-11(15)16-9/h2-6H,1H3,(H,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NAT1 assessed as hydrolysis of acetyl coA using PABA as substrate by Ellman's method |
Bioorg Med Chem 17: 905-18 (2009)
Article DOI: 10.1016/j.bmc.2008.11.032
BindingDB Entry DOI: 10.7270/Q2PZ58Q3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data