

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59213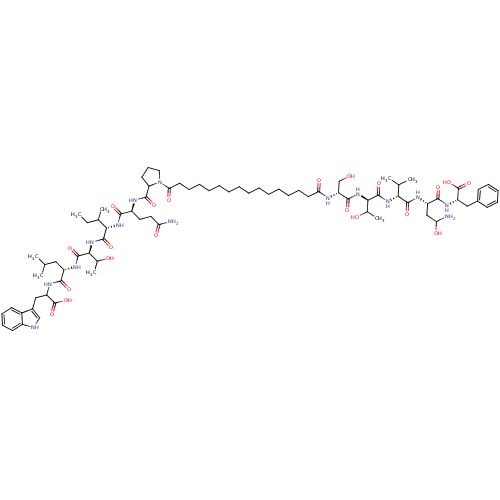 (Pepstatin analog, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | 350 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59224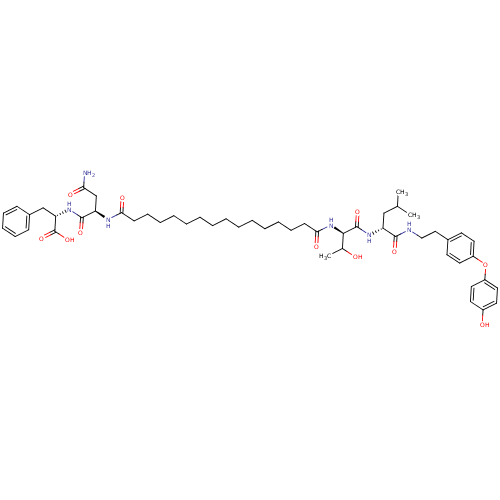 (Pepstatin analog, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | 360 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50289659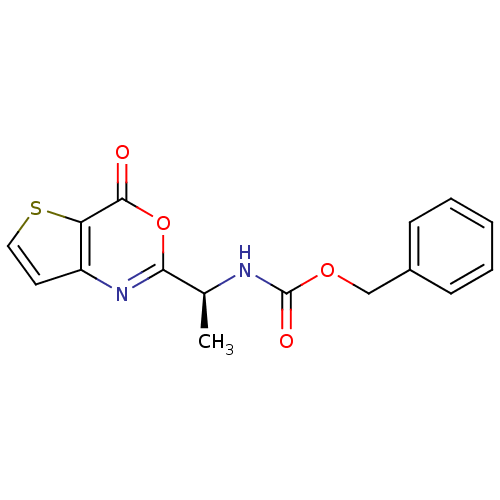 (CHEMBL49336 | [(S)-1-(4-Oxo-4H-thieno[3,2-d][1,3]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50289663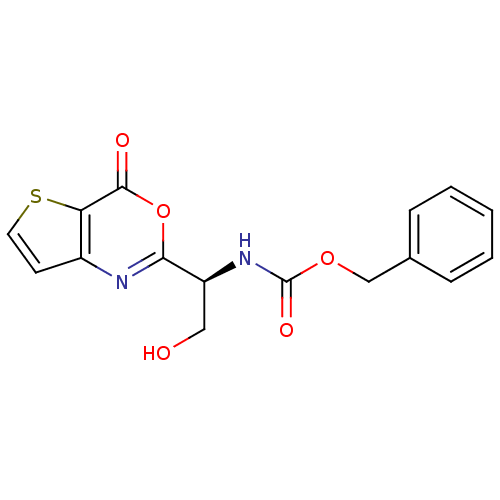 (CHEMBL299316 | [(S)-2-Hydroxy-1-(4-oxo-4H-thieno[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59223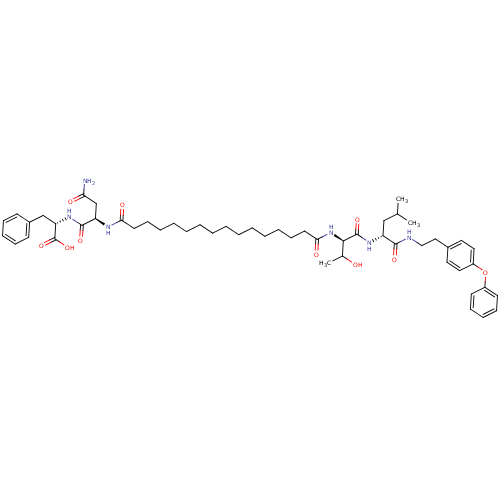 (Pepstatin analog, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 175 | n/a | 730 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288088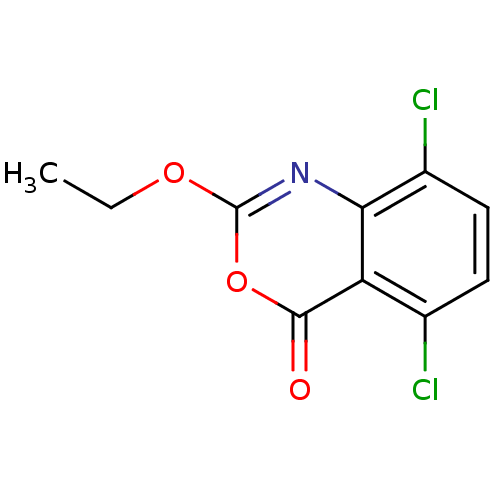 (5,8-Dichloro-2-ethoxy-benzo[d][1,3]oxazin-4-one | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59222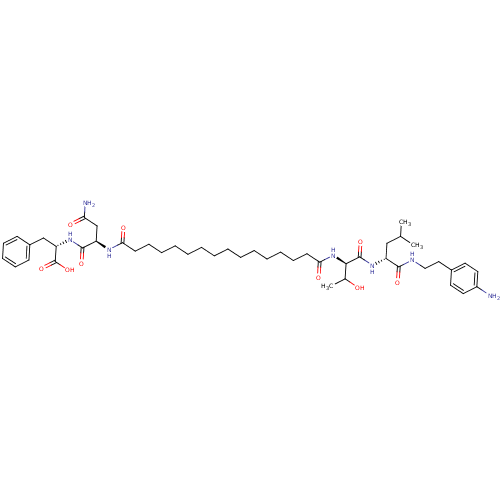 (Pepstatin analog, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288091 (2-(4-Methoxy-phenoxy)-benzo[d][1,3]oxazin-4-one | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59219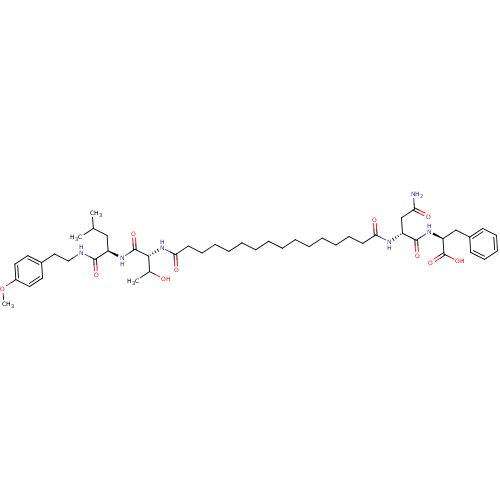 (Pepstatin analog, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 720 | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50289665 ((4-Oxo-4H-thieno[3,2-d][1,3]oxazin-2-ylmethyl)-car...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59220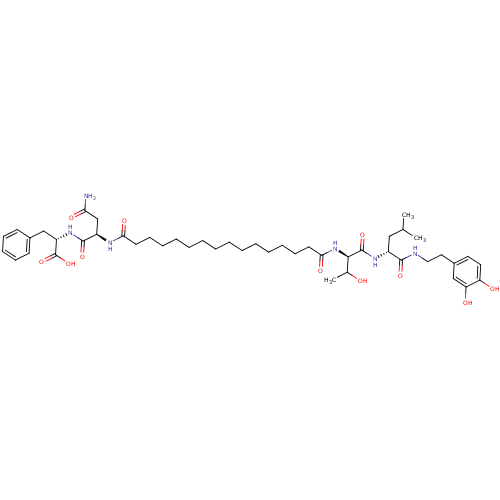 (Pepstatin analog, 8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50289660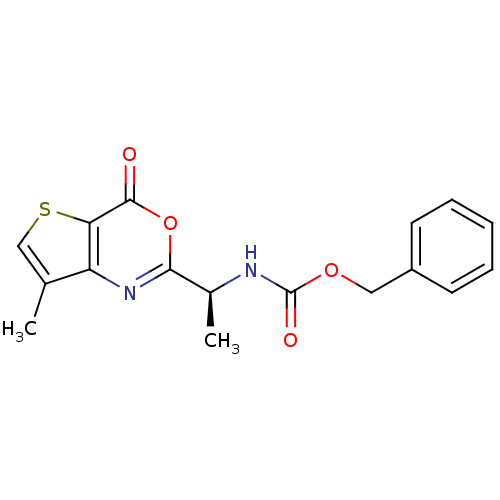 (CHEMBL49670 | [(S)-1-(7-Methyl-4-oxo-4H-thieno[3,2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50289664 (CHEMBL299594 | [(S)-1-(4-Oxo-6-phenyl-4H-thieno[3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59221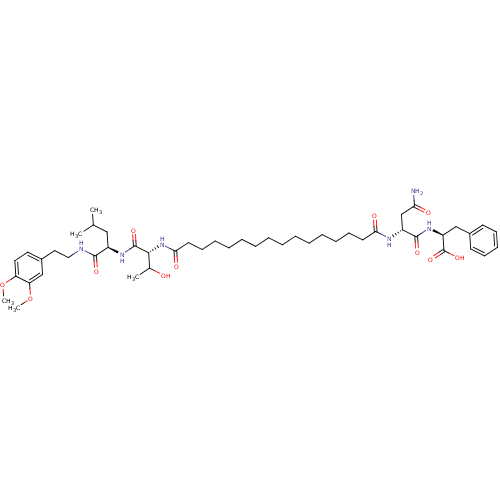 (Pepstatin analog, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59216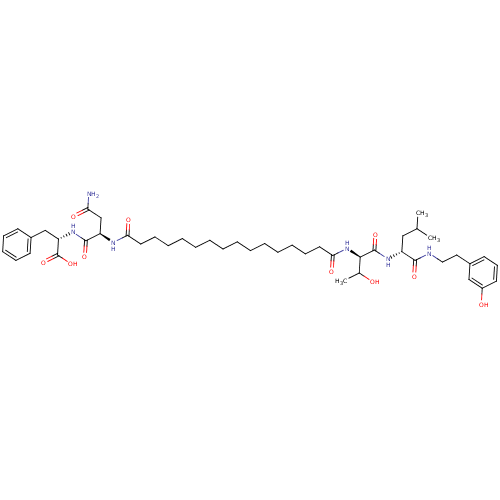 (Pepstatin analog, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.13E+3 | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59217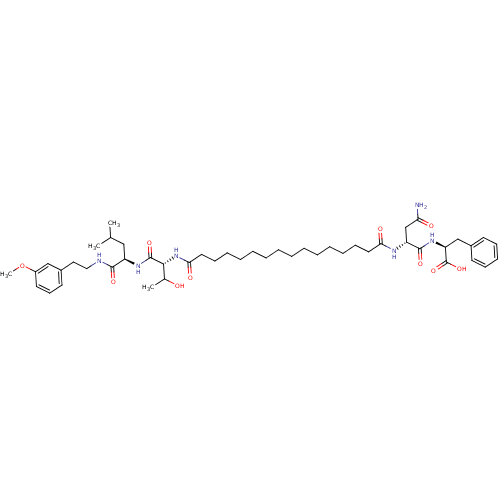 (Pepstatin analog, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.29E+3 | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288097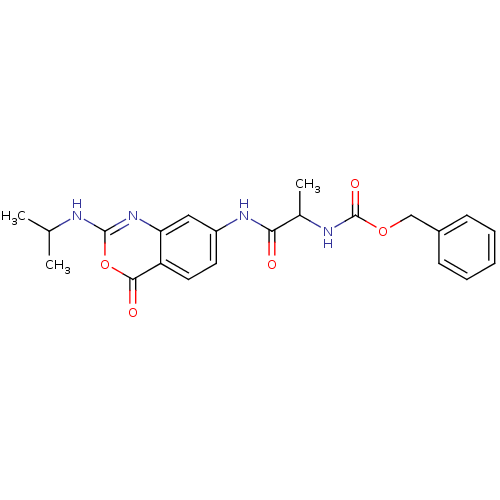 (CHEMBL81237 | [1-(2-Isopropylamino-4-oxo-4H-benzo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59218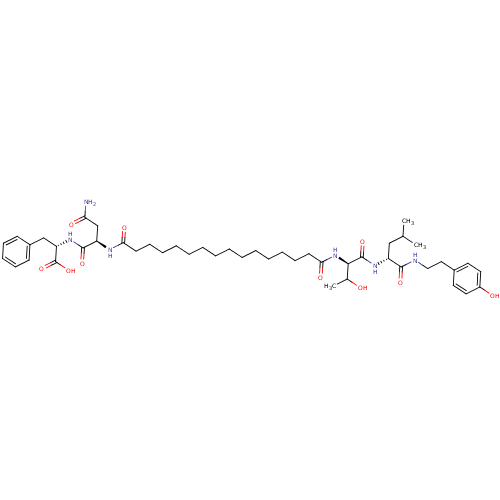 (Pepstatin analog, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description A zhang-poorman assay confirms a competitive inhibition mechanism or inhibit HIV-1 PR as a dimerization or mixed type inhibitor. | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59215 (Pepstatin analog, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM59214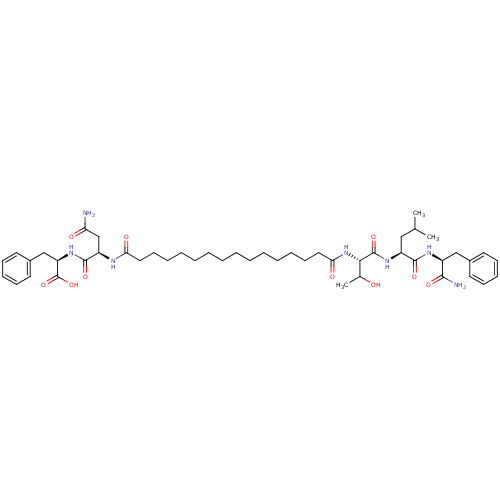 (Pepstatin analog, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | 5.52 | n/a |
Purdue University | Assay Description The competitive assay requires two inhibitors to act by a purely competitive mechanism, whereas the binding site of on the inhibitors has been establ... | Chem Biol 12: 439-44 (2005) Article DOI: 10.1016/j.chembiol.2005.02.004 BindingDB Entry DOI: 10.7270/Q2JD4V68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50289666 (CHEMBL47729 | [(S)-3-Carbamoyl-1-(4-oxo-4H-thieno[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288096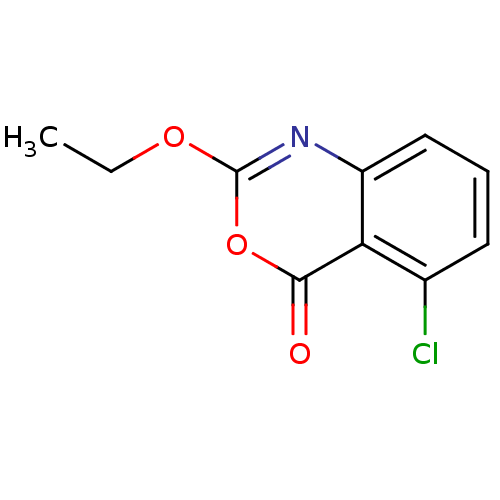 (5-Chloro-2-ethoxy-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288086 (2-Phenoxy-benzo[d][1,3]oxazin-4-one | CHEMBL81468) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50289658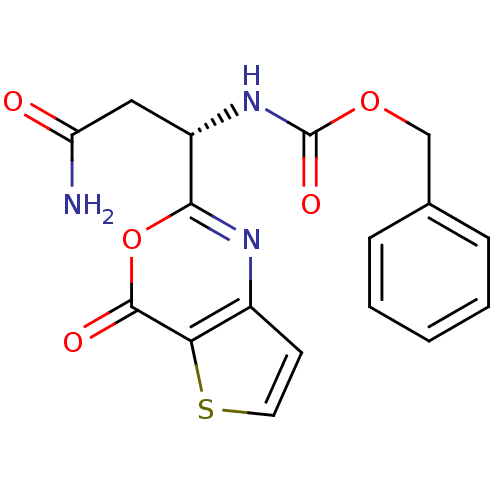 (CHEMBL45609 | [(S)-2-Carbamoyl-1-(4-oxo-4H-thieno[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288079 (CHEMBL79521 | {(S)-1-[7-(2-Benzyloxycarbonylamino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288082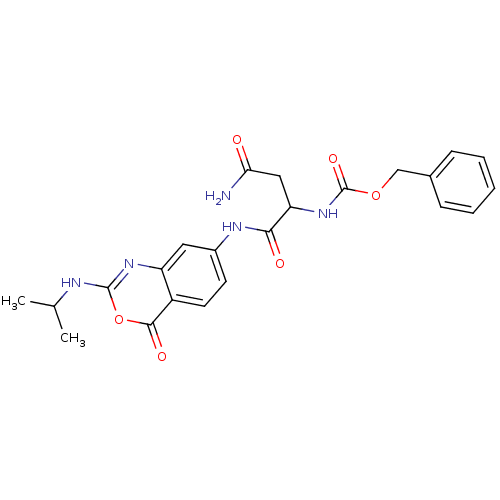 (CHEMBL79692 | [2-Carbamoyl-1-(2-isopropylamino-4-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288093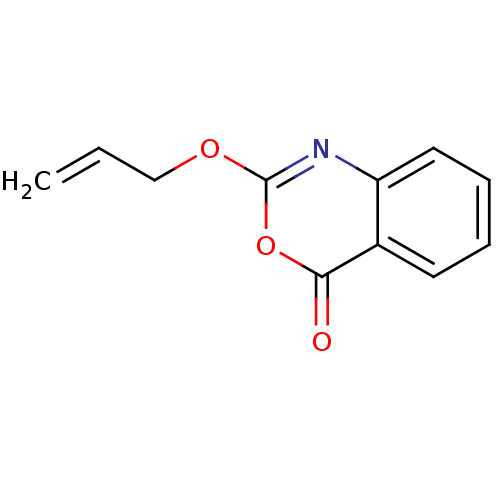 (2-Allyloxy-benzo[d][1,3]oxazin-4-one | CHEMBL81469) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288087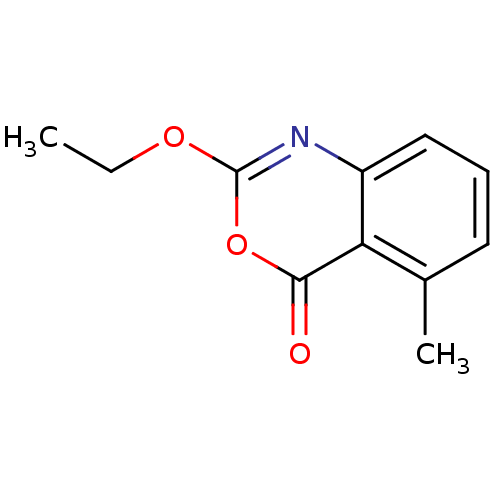 (2-Ethoxy-5-methyl-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288098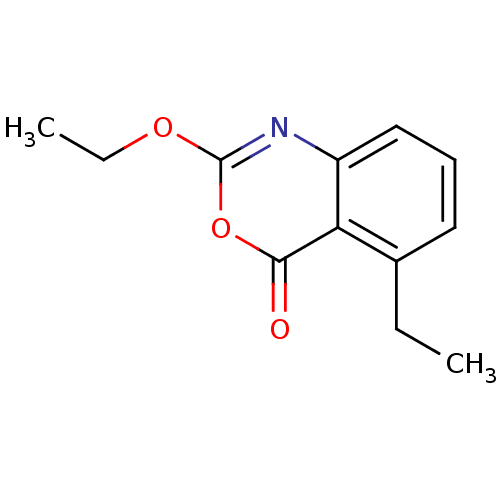 (2-Ethoxy-5-ethyl-benzo[d][1,3]oxazin-4-one | CHEMB...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288083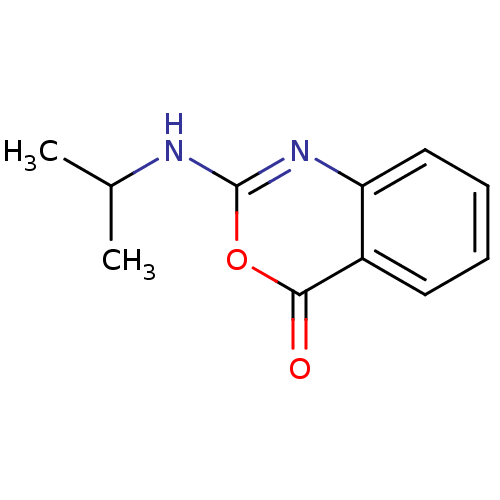 (2-Isopropylamino-benzo[d][1,3]oxazin-4-one | CHEMB...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288095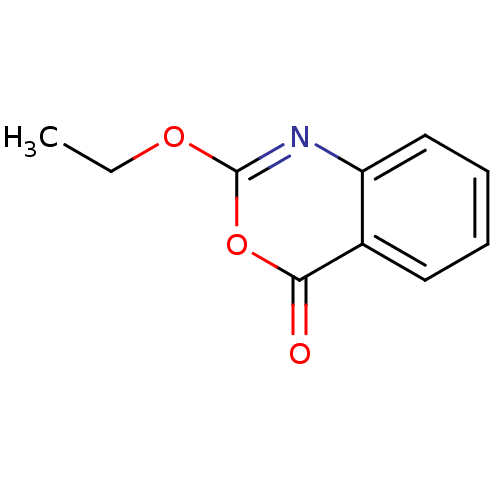 (2-Ethoxy-benzo[d][1,3]oxazin-4-one | CHEMBL450273) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288089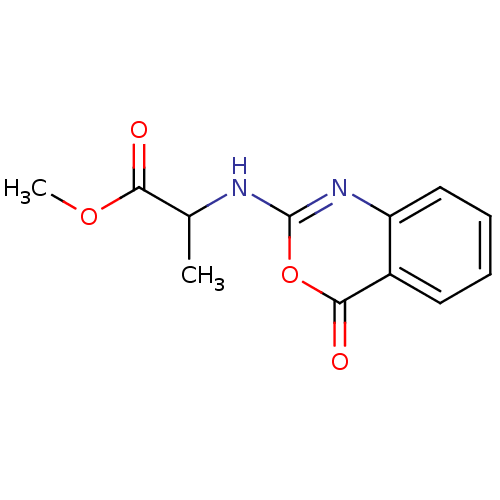 (2-(4-Oxo-4H-benzo[d][1,3]oxazin-2-ylamino)-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50289661 (CHEMBL45791 | [(S)-2-Methyl-1-(4-oxo-4H-thieno[3,2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288092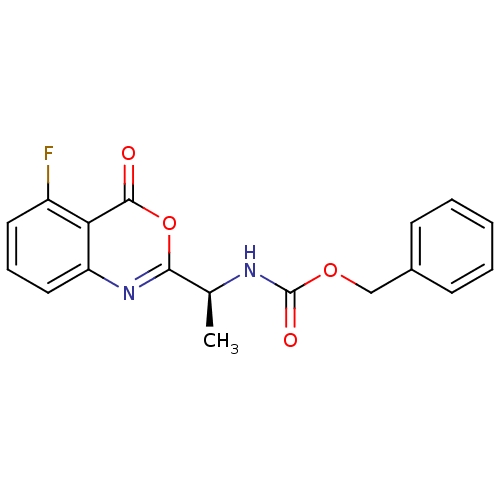 (CHEMBL83375 | [(S)-1-(5-Fluoro-4-oxo-4H-benzo[d][1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288084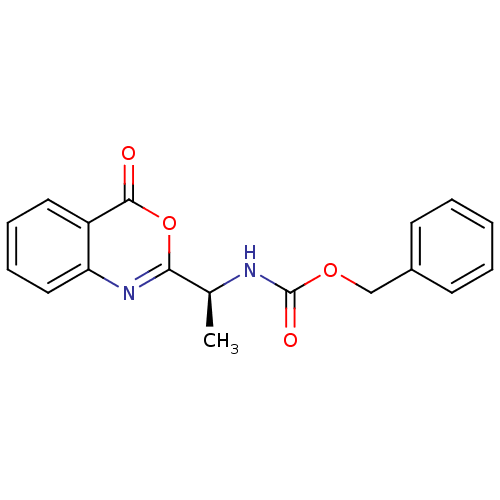 (CHEMBL49391 | [(S)-1-(4-Oxo-4H-benzo[d][1,3]oxazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288084 (CHEMBL49391 | [(S)-1-(4-Oxo-4H-benzo[d][1,3]oxazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288081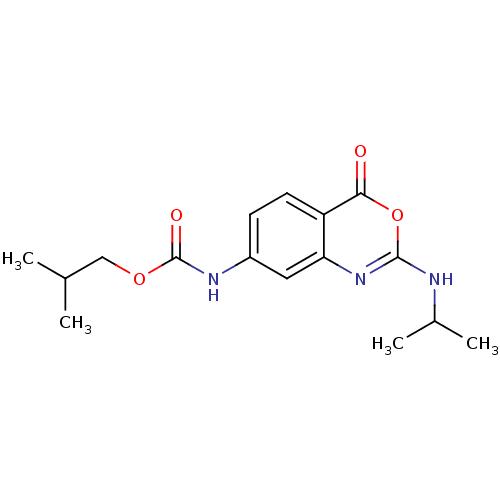 ((2-Isopropylamino-4-oxo-4H-benzo[d][1,3]oxazin-7-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288085 (7-Amino-2-isopropylamino-benzo[d][1,3]oxazin-4-one...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288103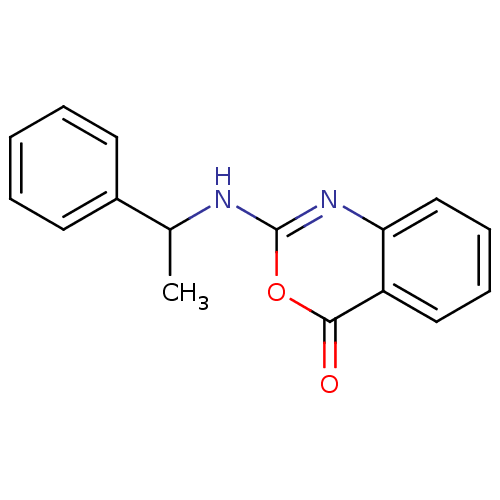 (2-(1-Phenyl-ethylamino)-benzo[d][1,3]oxazin-4-one ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288099 (2-Ethoxy-5-fluoro-benzo[d][1,3]oxazin-4-one | CHEM...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288102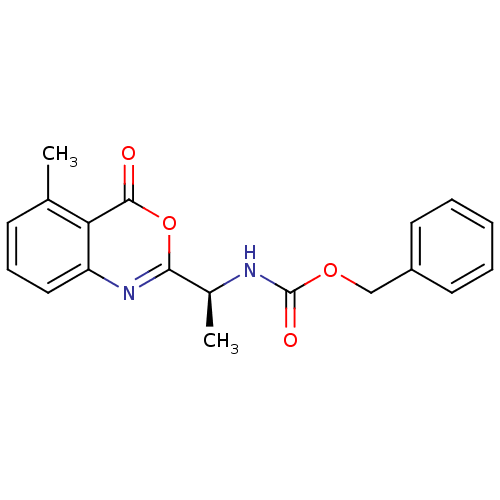 (CHEMBL45496 | [(S)-1-(5-Methyl-4-oxo-4H-benzo[d][1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288102 (CHEMBL45496 | [(S)-1-(5-Methyl-4-oxo-4H-benzo[d][1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288100 (CHEMBL316027 | [(S)-1-(7-Amino-4-oxo-4H-benzo[d][1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288094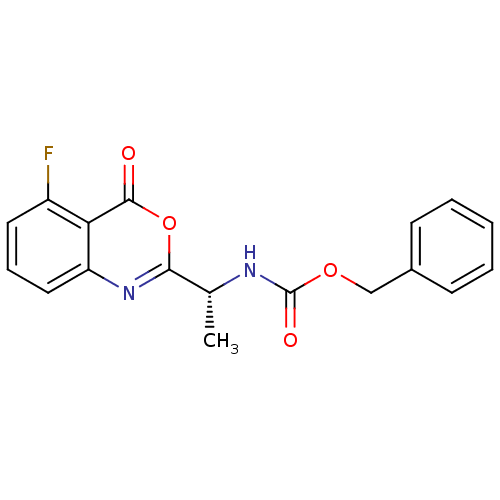 (CHEMBL421137 | [(R)-1-(5-Fluoro-4-oxo-4H-benzo[d][...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50289662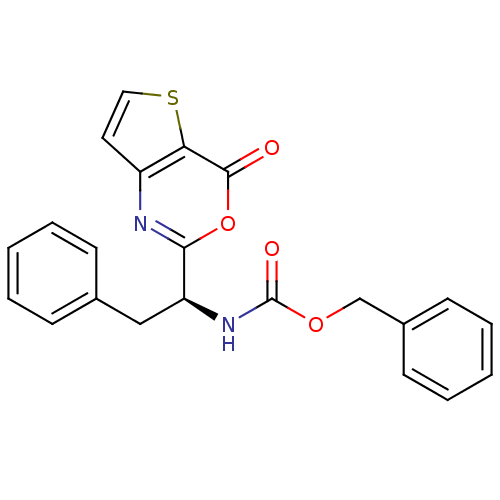 (CHEMBL301380 | [(S)-1-(4-Oxo-4H-thieno[3,2-d][1,3]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration for peptidolytic activity against herpes simplex type-1 (HSV-1) protease at 10 uM. | Bioorg Med Chem Lett 7: 1733-1738 (1997) Article DOI: 10.1016/S0960-894X(97)00300-4 BindingDB Entry DOI: 10.7270/Q23778RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288078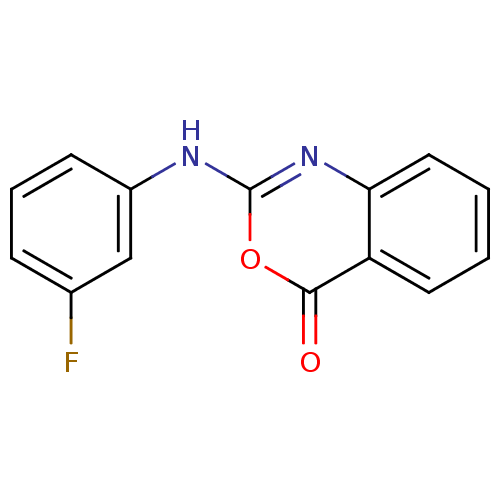 (2-(3-Fluoro-phenylamino)-benzo[d][1,3]oxazin-4-one...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288080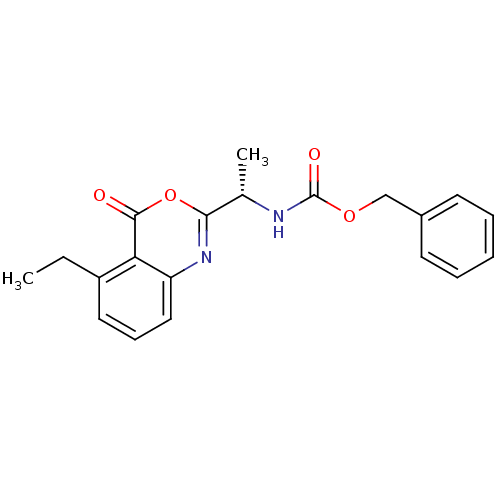 (CHEMBL441994 | [(S)-1-(5-Ethyl-4-oxo-4H-benzo[d][1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288101 (2-Butylamino-benzo[d][1,3]oxazin-4-one | CHEMBL799...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Capsid scaffolding protein (Human herpesvirus 1 (strain 17) (HHV-1) (Human her...) | BDBM50288090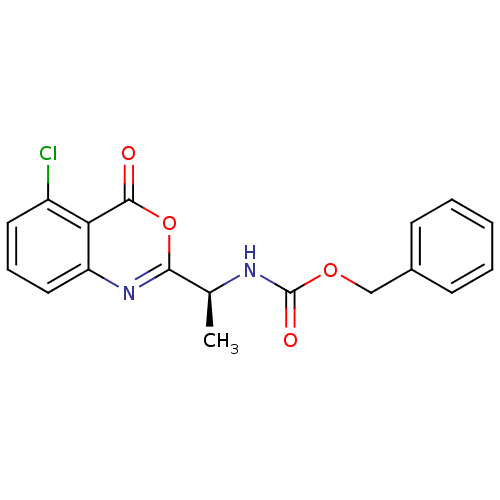 (CHEMBL79772 | [(S)-1-(5-Chloro-4-oxo-4H-benzo[d][1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against herpes simplex type 1 protease (HSV-1 pr). | Bioorg Med Chem Lett 6: 2463-2466 (1996) Article DOI: 10.1016/0960-894X(96)00455-6 BindingDB Entry DOI: 10.7270/Q2F47P4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||