 Found 53 hits of ki for UniProtKB: P20789
Found 53 hits of ki for UniProtKB: P20789 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]neurotensin from rat NTS1 receptor expressed in CHO-K1 cells by competitive binding assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM85050
(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]neurotensin from rat NTS1 receptor expressed in CHO-K1 cells by competitive binding assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50248034
(2-{[1-(7-Chloro-quinolin-4-yl)-5-(2,6-dimethoxy-ph...)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:38:37:35:31.32.33,39:29:35:31.32.33,28:29:31.38.32:36.34.35,THB:38:32:29.37.36:35,39:29:31.38.32:36.34.35,28:29:35:31.32.33,33:32:29:36.34.35,33:34:29:31.38.32,(-7.63,.4,;-6.3,-.36,;-6.29,-1.9,;-7.62,-2.68,;-7.62,-4.22,;-6.29,-4.99,;-4.95,-4.22,;-3.61,-4.99,;-3.61,-6.53,;-4.95,-2.67,;-3.63,-1.9,;-2.22,-2.52,;-1.19,-1.37,;-1.97,-.04,;-3.47,-.36,;-4.62,.66,;-6.08,.17,;-7.22,1.19,;-6.91,2.71,;-5.45,3.19,;-5.14,4.69,;-3.68,5.16,;-3.37,6.67,;-2.53,4.14,;-2.85,2.64,;-4.31,2.16,;.35,-1.53,;.98,-2.93,;1.24,-.27,;2.78,-.43,;3.43,1.2,;4.84,1.23,;6.02,2.07,;5.46,3.5,;3.95,3.52,;2.87,2.58,;3.44,1.99,;4.02,.52,;5.54,.51,;3.42,-1.84,;2.51,-3.09,;4.95,-2,)| Show InChI InChI=1S/C32H31ClN4O5/c1-41-27-4-3-5-28(42-2)29(27)26-16-24(36-37(26)25-8-9-34-23-15-21(33)6-7-22(23)25)30(38)35-32(31(39)40)19-11-17-10-18(13-19)14-20(32)12-17/h3-9,15-20H,10-14H2,1-2H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Durham University
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT from rat NTR1 transfected in mouse LTK cells |
Bioorg Med Chem 21: 4378-87 (2013)
Article DOI: 10.1016/j.bmc.2013.04.075
BindingDB Entry DOI: 10.7270/Q27M09B6 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50041427
(CHEMBL3356853)Show SMILES COc1cccc(OC)c1-c1nc(nc2ccc(Cl)cc12)C(=O)NC1(CCCCC1)C(O)=O |(41.35,-22.47,;42.69,-23.24,;42.69,-24.79,;41.36,-25.56,;41.36,-27.11,;42.7,-27.88,;44.04,-27.1,;45.37,-27.87,;45.38,-29.42,;44.03,-25.55,;45.36,-24.78,;46.7,-25.55,;48.03,-24.77,;48.03,-23.23,;46.67,-22.47,;46.66,-20.94,;45.33,-20.18,;44.01,-20.96,;42.66,-20.21,;44.02,-22.47,;45.35,-23.24,;49.37,-25.54,;49.38,-27.08,;50.71,-24.76,;52.04,-25.53,;53.38,-26.3,;54.7,-25.53,;54.71,-23.99,;53.38,-23.22,;52.04,-23.99,;52.05,-27.08,;50.72,-27.85,;53.39,-27.84,)| Show InChI InChI=1S/C24H24ClN3O5/c1-32-17-7-6-8-18(33-2)19(17)20-15-13-14(25)9-10-16(15)26-21(27-20)22(29)28-24(23(30)31)11-4-3-5-12-24/h6-10,13H,3-5,11-12H2,1-2H3,(H,28,29)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50041429
(CHEMBL3356854)Show SMILES COc1cccc(OC)c1-c1nc(nc2ccc(Cl)cc12)C(=O)N[C@@H](CC(C)C)C(O)=O |r,wU:24.27,(2.86,-22.1,;4.19,-22.87,;4.2,-24.41,;2.87,-25.18,;2.87,-26.72,;4.2,-27.49,;5.54,-26.72,;6.87,-27.49,;6.87,-29.03,;5.53,-25.17,;6.86,-24.4,;8.19,-25.17,;9.52,-24.39,;9.52,-22.85,;8.17,-22.09,;8.16,-20.57,;6.83,-19.81,;5.51,-20.59,;4.17,-19.84,;5.52,-22.09,;6.85,-22.87,;10.86,-25.16,;10.87,-26.7,;12.19,-24.38,;13.53,-25.15,;14.86,-24.38,;16.2,-25.14,;17.53,-24.37,;16.2,-26.68,;13.54,-26.69,;12.2,-27.47,;14.87,-27.46,)| Show InChI InChI=1S/C23H24ClN3O5/c1-12(2)10-16(23(29)30)26-22(28)21-25-15-9-8-13(24)11-14(15)20(27-21)19-17(31-3)6-5-7-18(19)32-4/h5-9,11-12,16H,10H2,1-4H3,(H,26,28)(H,29,30)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50041428

(CHEMBL3356855)Show SMILES COc1cccc(OC)c1-c1nc(nc2ccc(Cl)cc12)C(=O)N[C@@H](C1CCCCC1)C(O)=O |r,wU:24.27,(22.57,-23.73,;23.91,-24.49,;23.91,-26.03,;22.58,-26.8,;22.58,-28.35,;23.92,-29.12,;25.25,-28.35,;26.59,-29.11,;26.59,-30.65,;25.25,-26.8,;26.57,-26.02,;27.91,-26.79,;29.24,-26.02,;29.24,-24.48,;27.88,-23.72,;27.87,-22.19,;26.55,-21.44,;25.22,-22.22,;23.88,-21.46,;25.24,-23.72,;26.57,-24.49,;30.58,-26.78,;30.58,-28.32,;31.91,-26.01,;33.24,-26.77,;34.58,-26,;35.91,-26.77,;37.24,-26.01,;37.24,-24.46,;35.91,-23.69,;34.57,-24.47,;33.25,-28.32,;31.92,-29.09,;34.59,-29.08,)| Show InChI InChI=1S/C25H26ClN3O5/c1-33-18-9-6-10-19(34-2)20(18)22-16-13-15(26)11-12-17(16)27-23(28-22)24(30)29-21(25(31)32)14-7-4-3-5-8-14/h6,9-14,21H,3-5,7-8H2,1-2H3,(H,29,30)(H,31,32)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366375
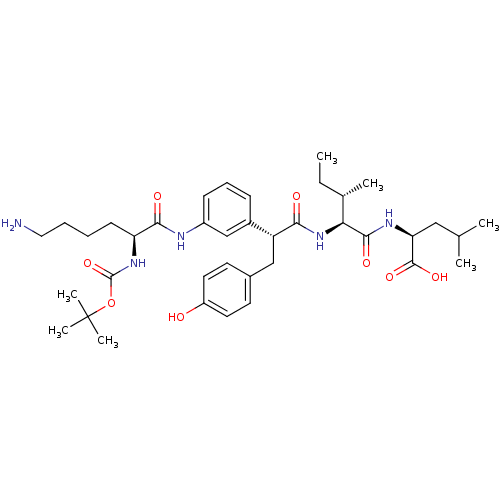
(CHEMBL1794002)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)c1cccc(NC(=O)[C@H](CCCCN)NC(=O)OC(C)(C)C)c1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C38H57N5O8/c1-8-24(4)32(35(47)41-31(36(48)49)20-23(2)3)43-33(45)29(21-25-15-17-28(44)18-16-25)26-12-11-13-27(22-26)40-34(46)30(14-9-10-19-39)42-37(50)51-38(5,6)7/h11-13,15-18,22-24,29-32,44H,8-10,14,19-21,39H2,1-7H3,(H,40,46)(H,41,47)(H,42,50)(H,43,45)(H,48,49)/t24-,29+,30-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against Neurotensin receptor |
Bioorg Med Chem Lett 5: 1203-1206 (1995)
Article DOI: 10.1016/0960-894X(95)00195-Y
BindingDB Entry DOI: 10.7270/Q2RF5VHW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281809
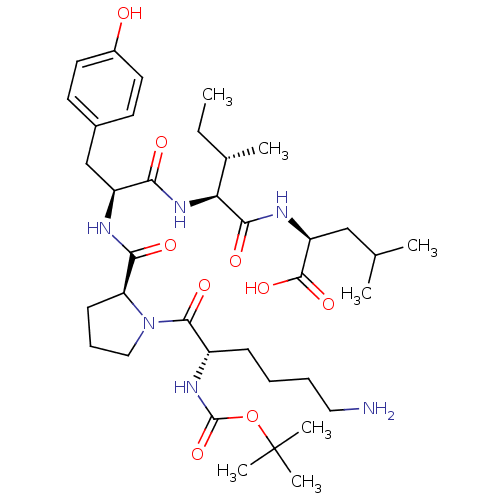
((S)-2-{(2S,3S)-2-[(S)-2-{[(S)-1-((S)-6-Amino-2-ter...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C37H60N6O9/c1-8-23(4)30(33(47)40-28(35(49)50)20-22(2)3)42-31(45)27(21-24-14-16-25(44)17-15-24)39-32(46)29-13-11-19-43(29)34(48)26(12-9-10-18-38)41-36(51)52-37(5,6)7/h14-17,22-23,26-30,44H,8-13,18-21,38H2,1-7H3,(H,39,46)(H,40,47)(H,41,51)(H,42,45)(H,49,50)/t23-,26-,27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281809

((S)-2-{(2S,3S)-2-[(S)-2-{[(S)-1-((S)-6-Amino-2-ter...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C37H60N6O9/c1-8-23(4)30(33(47)40-28(35(49)50)20-22(2)3)42-31(45)27(21-24-14-16-25(44)17-15-24)39-32(46)29-13-11-19-43(29)34(48)26(12-9-10-18-38)41-36(51)52-37(5,6)7/h14-17,22-23,26-30,44H,8-13,18-21,38H2,1-7H3,(H,39,46)(H,40,47)(H,41,51)(H,42,45)(H,49,50)/t23-,26-,27-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Neurotensin receptor binding affinity by displacement of [3H]- neurotensin |
Bioorg Med Chem Lett 3: 1035-1040 (1993)
Article DOI: 10.1016/S0960-894X(00)80282-6
BindingDB Entry DOI: 10.7270/Q27S7NQN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281812
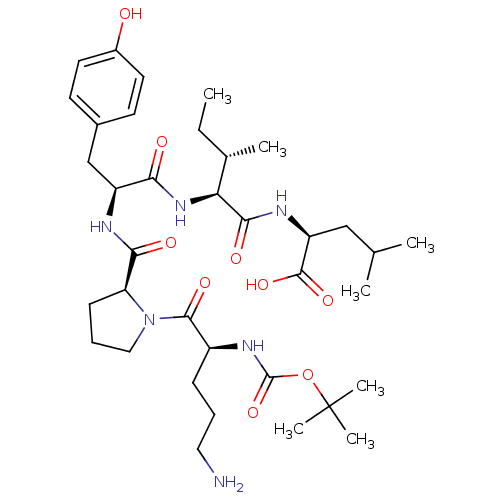
((S)-2-{(2S,3S)-2-[(S)-2-{[(S)-1-((S)-5-Amino-2-ter...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C36H58N6O9/c1-8-22(4)29(32(46)39-27(34(48)49)19-21(2)3)41-30(44)26(20-23-13-15-24(43)16-14-23)38-31(45)28-12-10-18-42(28)33(47)25(11-9-17-37)40-35(50)51-36(5,6)7/h13-16,21-22,25-29,43H,8-12,17-20,37H2,1-7H3,(H,38,45)(H,39,46)(H,40,50)(H,41,44)(H,48,49)/t22-,25-,26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281812

((S)-2-{(2S,3S)-2-[(S)-2-{[(S)-1-((S)-5-Amino-2-ter...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C36H58N6O9/c1-8-22(4)29(32(46)39-27(34(48)49)19-21(2)3)41-30(44)26(20-23-13-15-24(43)16-14-23)38-31(45)28-12-10-18-42(28)33(47)25(11-9-17-37)40-35(50)51-36(5,6)7/h13-16,21-22,25-29,43H,8-12,17-20,37H2,1-7H3,(H,38,45)(H,39,46)(H,40,50)(H,41,44)(H,48,49)/t22-,25-,26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Neurotensin receptor binding affinity by displacement of [3H]- neurotensin |
Bioorg Med Chem Lett 3: 1035-1040 (1993)
Article DOI: 10.1016/S0960-894X(00)80282-6
BindingDB Entry DOI: 10.7270/Q27S7NQN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366303
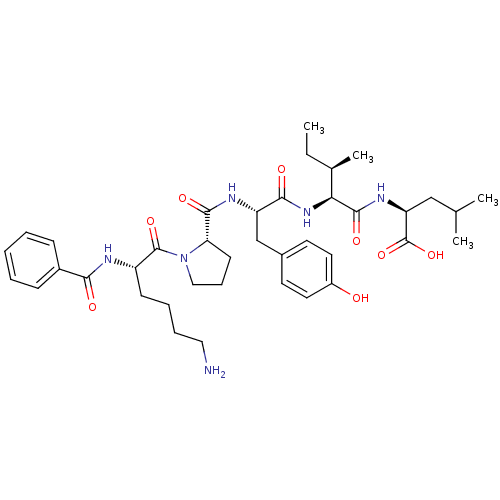
(CHEMBL1790460)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)c1ccccc1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C39H56N6O8/c1-5-25(4)33(37(50)43-31(39(52)53)22-24(2)3)44-35(48)30(23-26-16-18-28(46)19-17-26)42-36(49)32-15-11-21-45(32)38(51)29(14-9-10-20-40)41-34(47)27-12-7-6-8-13-27/h6-8,12-13,16-19,24-25,29-33,46H,5,9-11,14-15,20-23,40H2,1-4H3,(H,41,47)(H,42,49)(H,43,50)(H,44,48)(H,52,53)/t25-,29+,30+,31+,32+,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281811
((S)-2-{(2S,3S)-2-[(S)-2-[((S)-1-{(S)-2-[(Adamantan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)C12CC3CC(CC(C3)C1)C2)C(=O)N[C@@H](CC(C)C)C(O)=O |TLB:39:40:44:37.38.43,THB:41:40:37:44.42.43,41:42:37:45.40.39,39:38:44:45.40.41| Show InChI InChI=1S/C43H66N6O8/c1-5-26(4)36(39(53)46-34(41(55)56)17-25(2)3)48-37(51)33(21-27-11-13-31(50)14-12-27)45-38(52)35-10-8-16-49(35)40(54)32(9-6-7-15-44)47-42(57)43-22-28-18-29(23-43)20-30(19-28)24-43/h11-14,25-26,28-30,32-36,50H,5-10,15-24,44H2,1-4H3,(H,45,52)(H,46,53)(H,47,57)(H,48,51)(H,55,56)/t26-,28?,29?,30?,32-,33-,34-,35-,36-,43?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Neurotensin receptor binding affinity by displacement of [3H]- neurotensin |
Bioorg Med Chem Lett 3: 1035-1040 (1993)
Article DOI: 10.1016/S0960-894X(00)80282-6
BindingDB Entry DOI: 10.7270/Q27S7NQN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281811

((S)-2-{(2S,3S)-2-[(S)-2-[((S)-1-{(S)-2-[(Adamantan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)C12CC3CC(CC(C3)C1)C2)C(=O)N[C@@H](CC(C)C)C(O)=O |TLB:39:40:44:37.38.43,THB:41:40:37:44.42.43,41:42:37:45.40.39,39:38:44:45.40.41| Show InChI InChI=1S/C43H66N6O8/c1-5-26(4)36(39(53)46-34(41(55)56)17-25(2)3)48-37(51)33(21-27-11-13-31(50)14-12-27)45-38(52)35-10-8-16-49(35)40(54)32(9-6-7-15-44)47-42(57)43-22-28-18-29(23-43)20-30(19-28)24-43/h11-14,25-26,28-30,32-36,50H,5-10,15-24,44H2,1-4H3,(H,45,52)(H,46,53)(H,47,57)(H,48,51)(H,55,56)/t26-,28?,29?,30?,32-,33-,34-,35-,36-,43?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50019412
(CHEMBL3290096)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccnc2cc(Cl)ccc12)C(=O)NC1(CCCCC1)C(O)=O |(21.2,-17.42,;21.67,-15.96,;23.18,-15.64,;24.21,-16.78,;25.72,-16.46,;26.19,-15,;25.16,-13.85,;25.64,-12.39,;27.14,-12.07,;23.66,-14.17,;22.32,-12.69,;22.64,-11.18,;21.3,-10.41,;20.16,-11.44,;20.79,-12.85,;19.78,-14.58,;20.55,-15.92,;19.78,-17.25,;18.24,-17.25,;17.47,-15.92,;15.93,-15.92,;15.16,-14.58,;13.62,-14.58,;15.93,-13.25,;17.47,-13.25,;18.24,-14.58,;21.14,-8.88,;22.39,-7.97,;19.74,-8.25,;19.57,-6.72,;21.11,-6.83,;21.97,-5.55,;21.3,-4.17,;19.76,-4.06,;18.9,-5.34,;18.08,-7.16,;17,-6.07,;17.72,-8.64,)| Show InChI InChI=1S/C28H27ClN4O5/c1-37-23-7-6-8-24(38-2)25(23)22-16-20(26(34)31-28(27(35)36)12-4-3-5-13-28)32-33(22)21-11-14-30-19-15-17(29)9-10-18(19)21/h6-11,14-16H,3-5,12-13H2,1-2H3,(H,31,34)(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Displacement of [I125]neurotensin from rat NTS1 overexpressed in CHO-k1 cells by competitive binding Assay |
Bioorg Med Chem Lett 25: 2060-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.083
BindingDB Entry DOI: 10.7270/Q2BZ67RD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366296
(CHEMBL1790463)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)C12CC3CC(CC(C3)C1)C2)C(=O)N[C@@H](CC(C)C)C(O)=O |TLB:42:41:44:36.37.38,42:37:44:43.41.40,38:39:43:36.37.42,THB:38:37:43:44.39.40| Show InChI InChI=1S/C42H64N6O8/c1-5-25(4)35(38(52)45-33(40(54)55)16-24(2)3)47-36(50)32(20-26-10-12-30(49)13-11-26)44-37(51)34-9-7-15-48(34)39(53)31(8-6-14-43)46-41(56)42-21-27-17-28(22-42)19-29(18-27)23-42/h10-13,24-25,27-29,31-35,49H,5-9,14-23,43H2,1-4H3,(H,44,51)(H,45,52)(H,46,56)(H,47,50)(H,54,55)/t25-,27?,28?,29?,31+,32+,33+,34+,35+,42?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366308
(CHEMBL1790472)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)C(C)(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C37H60N6O8/c1-8-23(4)30(33(47)40-28(35(49)50)20-22(2)3)42-31(45)27(21-24-14-16-25(44)17-15-24)39-32(46)29-13-11-19-43(29)34(48)26(12-9-10-18-38)41-36(51)37(5,6)7/h14-17,22-23,26-30,44H,8-13,18-21,38H2,1-7H3,(H,39,46)(H,40,47)(H,41,51)(H,42,45)(H,49,50)/t23-,26+,27+,28+,29+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366299
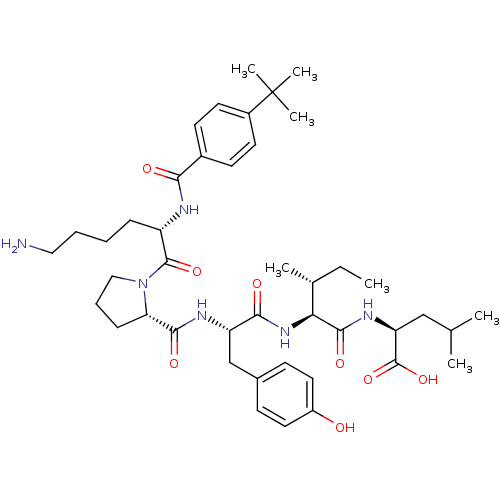
(CHEMBL1790467)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C43H64N6O8/c1-8-27(4)36(40(54)47-34(42(56)57)24-26(2)3)48-38(52)33(25-28-14-20-31(50)21-15-28)46-39(53)35-13-11-23-49(35)41(55)32(12-9-10-22-44)45-37(51)29-16-18-30(19-17-29)43(5,6)7/h14-21,26-27,32-36,50H,8-13,22-25,44H2,1-7H3,(H,45,51)(H,46,53)(H,47,54)(H,48,52)(H,56,57)/t27-,32+,33+,34+,35+,36+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366307
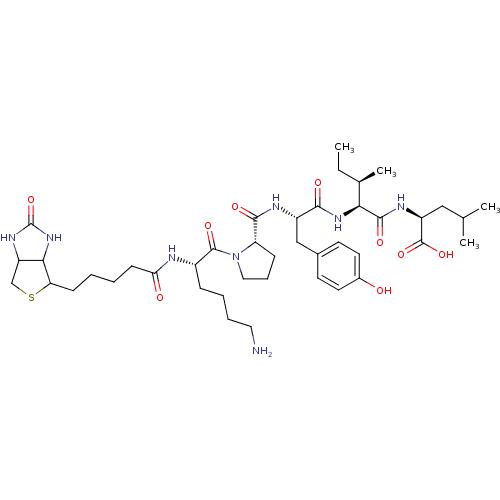
(CHEMBL1790457)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)CCCCC1SCC2NC(=O)NC12)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C42H66N8O9S/c1-5-25(4)35(39(55)46-30(41(57)58)21-24(2)3)48-37(53)29(22-26-15-17-27(51)18-16-26)45-38(54)32-12-10-20-50(32)40(56)28(11-8-9-19-43)44-34(52)14-7-6-13-33-36-31(23-60-33)47-42(59)49-36/h15-18,24-25,28-33,35-36,51H,5-14,19-23,43H2,1-4H3,(H,44,52)(H,45,54)(H,46,55)(H,48,53)(H,57,58)(H2,47,49,59)/t25-,28+,29+,30+,31?,32+,33?,35+,36?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366298
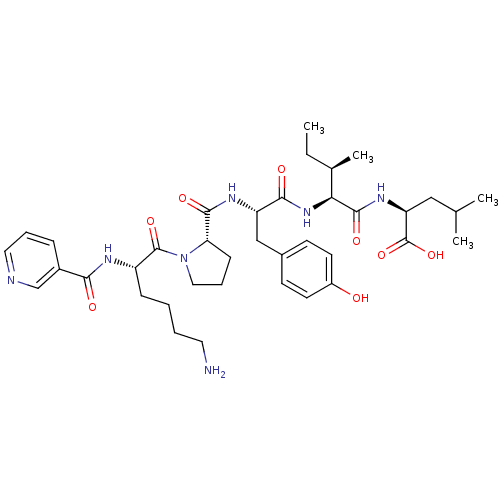
(CHEMBL1790466)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)c1cccnc1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C38H55N7O8/c1-5-24(4)32(36(50)43-30(38(52)53)20-23(2)3)44-34(48)29(21-25-13-15-27(46)16-14-25)42-35(49)31-12-9-19-45(31)37(51)28(11-6-7-17-39)41-33(47)26-10-8-18-40-22-26/h8,10,13-16,18,22-24,28-32,46H,5-7,9,11-12,17,19-21,39H2,1-4H3,(H,41,47)(H,42,49)(H,43,50)(H,44,48)(H,52,53)/t24-,28+,29+,30+,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366311
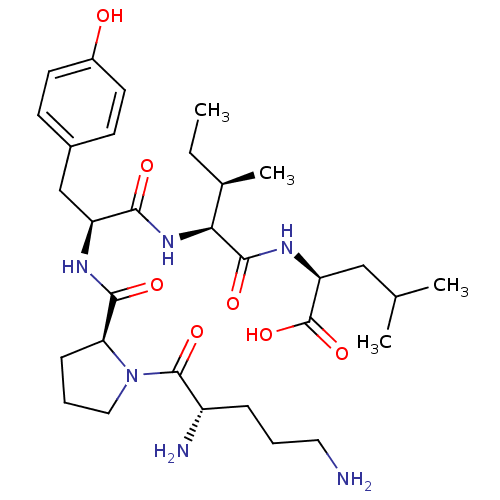
(CHEMBL1790470)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCN)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C31H50N6O7/c1-5-19(4)26(29(41)35-24(31(43)44)16-18(2)3)36-27(39)23(17-20-10-12-21(38)13-11-20)34-28(40)25-9-7-15-37(25)30(42)22(33)8-6-14-32/h10-13,18-19,22-26,38H,5-9,14-17,32-33H2,1-4H3,(H,34,40)(H,35,41)(H,36,39)(H,43,44)/t19-,22+,23+,24+,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366302
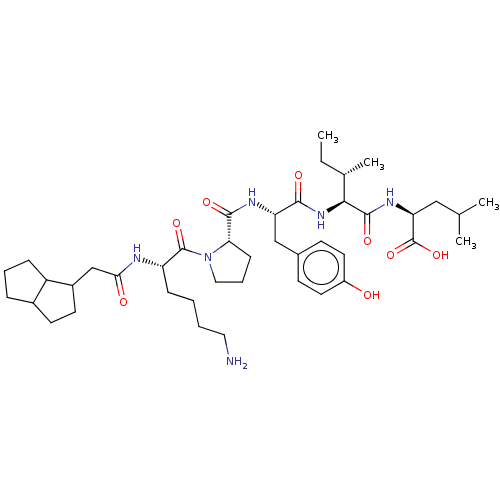
(CHEMBL2369587)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)CC1CCC2CCCC12)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C42H66N6O8/c1-5-26(4)37(40(53)46-34(42(55)56)22-25(2)3)47-38(51)33(23-27-14-18-30(49)19-15-27)45-39(52)35-13-9-21-48(35)41(54)32(12-6-7-20-43)44-36(50)24-29-17-16-28-10-8-11-31(28)29/h14-15,18-19,25-26,28-29,31-35,37,49H,5-13,16-17,20-24,43H2,1-4H3,(H,44,50)(H,45,52)(H,46,53)(H,47,51)(H,55,56)/t26-,28?,29?,31?,32-,33-,34-,35-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366310

(CHEMBL1790461)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)CC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C38H62N6O8/c1-8-24(4)32(35(49)42-29(37(51)52)20-23(2)3)43-33(47)28(21-25-14-16-26(45)17-15-25)41-34(48)30-13-11-19-44(30)36(50)27(12-9-10-18-39)40-31(46)22-38(5,6)7/h14-17,23-24,27-30,32,45H,8-13,18-22,39H2,1-7H3,(H,40,46)(H,41,48)(H,42,49)(H,43,47)(H,51,52)/t24-,27+,28+,29+,30+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281807
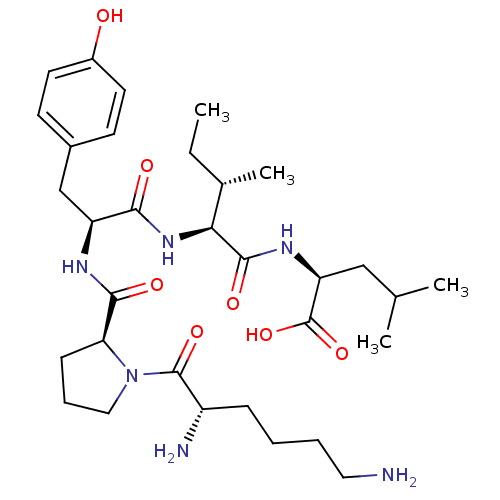
((S)-2-{(2S,3S)-2-[(S)-2-{[(S)-1-((S)-2,6-Diamino-h...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C32H52N6O7/c1-5-20(4)27(30(42)36-25(32(44)45)17-19(2)3)37-28(40)24(18-21-11-13-22(39)14-12-21)35-29(41)26-10-8-16-38(26)31(43)23(34)9-6-7-15-33/h11-14,19-20,23-27,39H,5-10,15-18,33-34H2,1-4H3,(H,35,41)(H,36,42)(H,37,40)(H,44,45)/t20-,23-,24-,25-,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 688 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Neurotensin receptor binding affinity by displacement of [3H]- neurotensin |
Bioorg Med Chem Lett 3: 1035-1040 (1993)
Article DOI: 10.1016/S0960-894X(00)80282-6
BindingDB Entry DOI: 10.7270/Q27S7NQN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281807

((S)-2-{(2S,3S)-2-[(S)-2-{[(S)-1-((S)-2,6-Diamino-h...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C32H52N6O7/c1-5-20(4)27(30(42)36-25(32(44)45)17-19(2)3)37-28(40)24(18-21-11-13-22(39)14-12-21)35-29(41)26-10-8-16-38(26)31(43)23(34)9-6-7-15-33/h11-14,19-20,23-27,39H,5-10,15-18,33-34H2,1-4H3,(H,35,41)(H,36,42)(H,37,40)(H,44,45)/t20-,23-,24-,25-,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366371
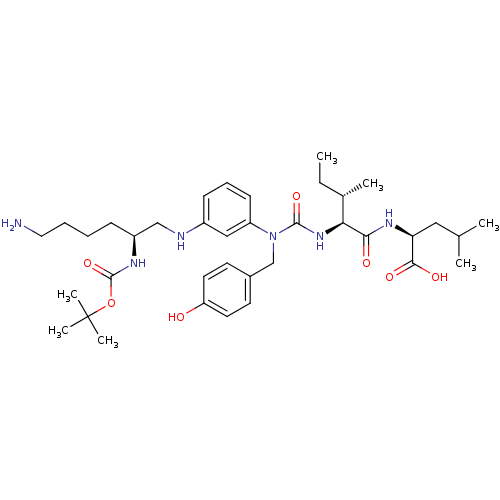
(CHEMBL1793833)Show SMILES CC[C@H](C)[C@H](NC(=O)N(Cc1ccc(O)cc1)c1cccc(NC[C@H](CCCCN)NC(=O)OC(C)(C)C)c1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C37H58N6O7/c1-8-25(4)32(33(45)41-31(34(46)47)20-24(2)3)42-35(48)43(23-26-15-17-30(44)18-16-26)29-14-11-13-27(21-29)39-22-28(12-9-10-19-38)40-36(49)50-37(5,6)7/h11,13-18,21,24-25,28,31-32,39,44H,8-10,12,19-20,22-23,38H2,1-7H3,(H,40,49)(H,41,45)(H,42,48)(H,46,47)/t25-,28-,31-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against Neurotensin receptor |
Bioorg Med Chem Lett 5: 1203-1206 (1995)
Article DOI: 10.1016/0960-894X(95)00195-Y
BindingDB Entry DOI: 10.7270/Q2RF5VHW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366300

(CHEMBL1790465)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)c1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H60N6O8/c1-5-29(4)39(43(56)49-37(45(58)59)26-28(2)3)50-41(54)36(27-30-16-22-34(52)23-17-30)48-42(55)38-15-11-25-51(38)44(57)35(14-9-10-24-46)47-40(53)33-20-18-32(19-21-33)31-12-7-6-8-13-31/h6-8,12-13,16-23,28-29,35-39,52H,5,9-11,14-15,24-27,46H2,1-4H3,(H,47,53)(H,48,55)(H,49,56)(H,50,54)(H,58,59)/t29-,35+,36+,37+,38+,39+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366374
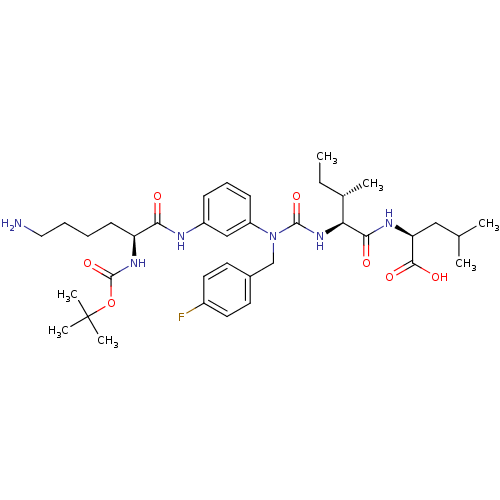
(CHEMBL1793999)Show SMILES CC[C@H](C)[C@H](NC(=O)N(Cc1ccc(F)cc1)c1cccc(NC(=O)[C@H](CCCCN)NC(=O)OC(C)(C)C)c1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C37H55FN6O7/c1-8-24(4)31(33(46)41-30(34(47)48)20-23(2)3)43-35(49)44(22-25-15-17-26(38)18-16-25)28-13-11-12-27(21-28)40-32(45)29(14-9-10-19-39)42-36(50)51-37(5,6)7/h11-13,15-18,21,23-24,29-31H,8-10,14,19-20,22,39H2,1-7H3,(H,40,45)(H,41,46)(H,42,50)(H,43,49)(H,47,48)/t24-,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against Neurotensin receptor |
Bioorg Med Chem Lett 5: 1203-1206 (1995)
Article DOI: 10.1016/0960-894X(95)00195-Y
BindingDB Entry DOI: 10.7270/Q2RF5VHW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366304
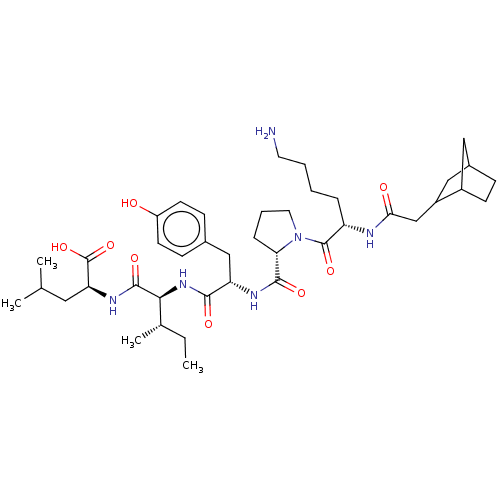
(CHEMBL2369588)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)CC1CC2CCC1C2)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C41H64N6O8/c1-5-25(4)36(39(52)45-33(41(54)55)19-24(2)3)46-37(50)32(22-26-12-15-30(48)16-13-26)44-38(51)34-10-8-18-47(34)40(53)31(9-6-7-17-42)43-35(49)23-29-21-27-11-14-28(29)20-27/h12-13,15-16,24-25,27-29,31-34,36,48H,5-11,14,17-23,42H2,1-4H3,(H,43,49)(H,44,51)(H,45,52)(H,46,50)(H,54,55)/t25-,27?,28?,29?,31-,32-,33-,34-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50019417
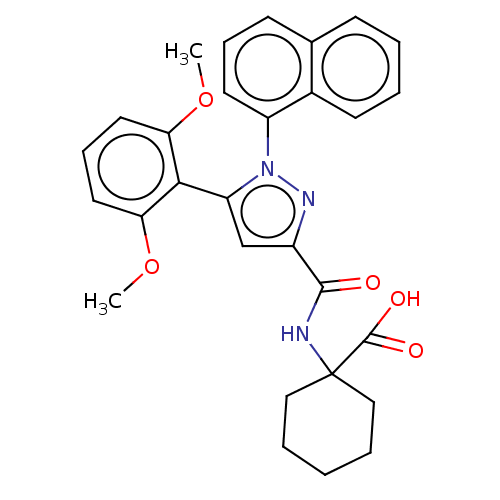
(CHEMBL3290101)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1cccc2ccccc12)C(=O)NC1(CCCCC1)C(O)=O |(20.81,-16.86,;21.28,-15.39,;22.79,-15.07,;23.82,-16.21,;25.33,-15.89,;25.8,-14.43,;24.77,-13.29,;25.25,-11.82,;26.75,-11.5,;23.27,-13.61,;21.93,-12.12,;22.25,-10.61,;20.91,-9.84,;19.77,-10.87,;20.39,-12.28,;19.39,-14.01,;20.16,-15.35,;19.39,-16.68,;17.85,-16.68,;17.08,-15.35,;15.54,-15.35,;14.77,-14.01,;15.54,-12.68,;17.08,-12.68,;17.85,-14.01,;20.75,-8.31,;22,-7.41,;19.34,-7.68,;19.18,-6.15,;20.72,-6.26,;21.58,-4.98,;20.91,-3.6,;19.37,-3.49,;18.51,-4.77,;17.69,-6.59,;17.33,-8.07,;16.6,-5.5,)| Show InChI InChI=1S/C29H29N3O5/c1-36-24-14-9-15-25(37-2)26(24)23-18-21(27(33)30-29(28(34)35)16-6-3-7-17-29)31-32(23)22-13-8-11-19-10-4-5-12-20(19)22/h4-5,8-15,18H,3,6-7,16-17H2,1-2H3,(H,30,33)(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Displacement of [I125]neurotensin from rat NTS1 overexpressed in CHO-k1 cells by competitive binding Assay |
Bioorg Med Chem Lett 25: 2060-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.083
BindingDB Entry DOI: 10.7270/Q2BZ67RD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366370
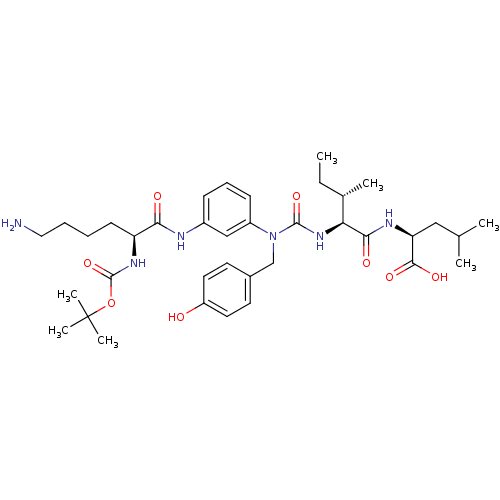
(CHEMBL1794003)Show SMILES CC[C@H](C)[C@H](NC(=O)N(Cc1ccc(O)cc1)c1cccc(NC(=O)[C@H](CCCCN)NC(=O)OC(C)(C)C)c1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C37H56N6O8/c1-8-24(4)31(33(46)40-30(34(47)48)20-23(2)3)42-35(49)43(22-25-15-17-28(44)18-16-25)27-13-11-12-26(21-27)39-32(45)29(14-9-10-19-38)41-36(50)51-37(5,6)7/h11-13,15-18,21,23-24,29-31,44H,8-10,14,19-20,22,38H2,1-7H3,(H,39,45)(H,40,46)(H,41,50)(H,42,49)(H,47,48)/t24-,29-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against Neurotensin receptor |
Bioorg Med Chem Lett 5: 1203-1206 (1995)
Article DOI: 10.1016/0960-894X(95)00195-Y
BindingDB Entry DOI: 10.7270/Q2RF5VHW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281806
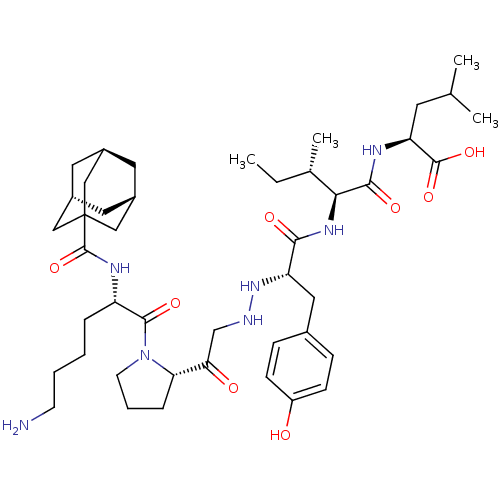
((S)-2-{(2S,3S)-2-[(S)-2-{N'-[2-((S)-1-{(S)-2-[(Ada...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NNCC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)C12C[C@H]3C[C@H](C[C@H](C3)C1)C2)C(=O)N[C@@H](CC(C)C)C(O)=O |TLB:41:42:46:39.40.45,THB:41:40:46:47.42.43| Show InChI InChI=1S/C44H69N7O8/c1-5-27(4)38(40(55)47-35(42(57)58)17-26(2)3)49-39(54)34(21-28-11-13-32(52)14-12-28)50-46-25-37(53)36-10-8-16-51(36)41(56)33(9-6-7-15-45)48-43(59)44-22-29-18-30(23-44)20-31(19-29)24-44/h11-14,26-27,29-31,33-36,38,46,50,52H,5-10,15-25,45H2,1-4H3,(H,47,55)(H,48,59)(H,49,54)(H,57,58)/t27-,29-,30+,31-,33-,34-,35-,36-,38-,44?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Neurotensin receptor binding affinity by displacement of [3H]- neurotensin |
Bioorg Med Chem Lett 3: 1035-1040 (1993)
Article DOI: 10.1016/S0960-894X(00)80282-6
BindingDB Entry DOI: 10.7270/Q27S7NQN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281810
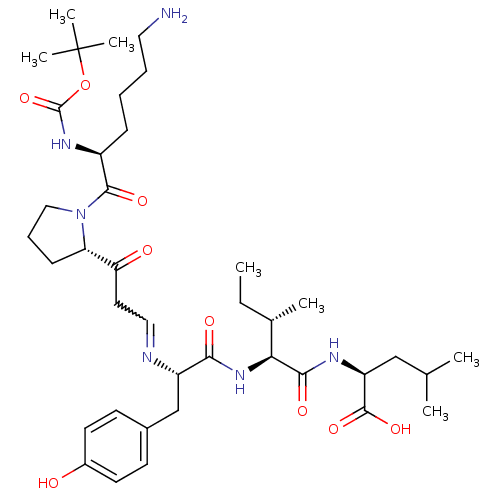
((S)-2-{(2S,3S)-2-[(S)-2-{(E)-3-[(S)-1-((S)-6-Amino...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)N=CCC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O |w:18.19| Show InChI InChI=1S/C39H62N6O9/c1-8-25(4)33(35(49)42-30(37(51)52)22-24(2)3)44-34(48)29(23-26-14-16-27(46)17-15-26)41-20-18-32(47)31-13-11-21-45(31)36(50)28(12-9-10-19-40)43-38(53)54-39(5,6)7/h14-17,20,24-25,28-31,33,46H,8-13,18-19,21-23,40H2,1-7H3,(H,42,49)(H,43,53)(H,44,48)(H,51,52)/t25-,28-,29-,30-,31-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Neurotensin receptor binding affinity by displacement of [3H]- neurotensin |
Bioorg Med Chem Lett 3: 1035-1040 (1993)
Article DOI: 10.1016/S0960-894X(00)80282-6
BindingDB Entry DOI: 10.7270/Q27S7NQN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366301
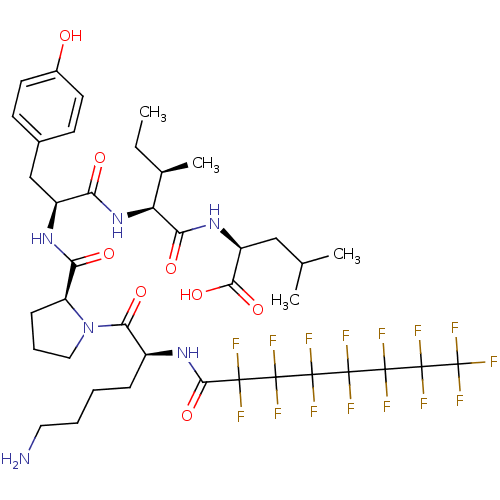
(CHEMBL1790469)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C40H51F15N6O8/c1-5-20(4)27(30(65)58-25(32(67)68)17-19(2)3)60-28(63)24(18-21-11-13-22(62)14-12-21)57-29(64)26-10-8-16-61(26)31(66)23(9-6-7-15-56)59-33(69)34(41,42)35(43,44)36(45,46)37(47,48)38(49,50)39(51,52)40(53,54)55/h11-14,19-20,23-27,62H,5-10,15-18,56H2,1-4H3,(H,57,64)(H,58,65)(H,59,69)(H,60,63)(H,67,68)/t20-,23+,24+,25+,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366306
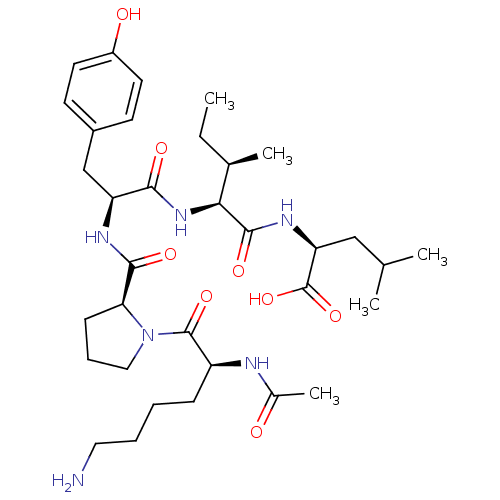
(CHEMBL1790473)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(C)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C34H54N6O8/c1-6-21(4)29(32(45)38-27(34(47)48)18-20(2)3)39-30(43)26(19-23-12-14-24(42)15-13-23)37-31(44)28-11-9-17-40(28)33(46)25(36-22(5)41)10-7-8-16-35/h12-15,20-21,25-29,42H,6-11,16-19,35H2,1-5H3,(H,36,41)(H,37,44)(H,38,45)(H,39,43)(H,47,48)/t21-,25+,26+,27+,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366373
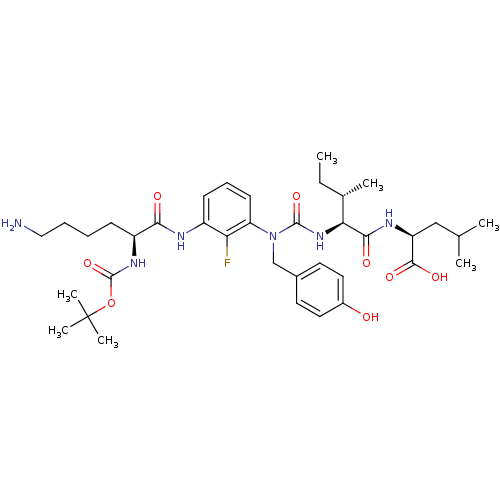
(CHEMBL1794000)Show SMILES CC[C@H](C)[C@H](NC(=O)N(Cc1ccc(O)cc1)c1cccc(NC(=O)[C@H](CCCCN)NC(=O)OC(C)(C)C)c1F)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C37H55FN6O8/c1-8-23(4)31(33(47)41-28(34(48)49)20-22(2)3)43-35(50)44(21-24-15-17-25(45)18-16-24)29-14-11-13-26(30(29)38)40-32(46)27(12-9-10-19-39)42-36(51)52-37(5,6)7/h11,13-18,22-23,27-28,31,45H,8-10,12,19-21,39H2,1-7H3,(H,40,46)(H,41,47)(H,42,51)(H,43,50)(H,48,49)/t23-,27-,28-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against Neurotensin receptor |
Bioorg Med Chem Lett 5: 1203-1206 (1995)
Article DOI: 10.1016/0960-894X(95)00195-Y
BindingDB Entry DOI: 10.7270/Q2RF5VHW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281804
((S)-2-{(2S,3S)-2-[(S)-2-(N'-{2-[(S)-1-((S)-5-Amino...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NNCC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C37H61N7O9/c1-8-23(4)31(33(48)40-28(35(50)51)19-22(2)3)42-32(47)27(20-24-13-15-25(45)16-14-24)43-39-21-30(46)29-12-10-18-44(29)34(49)26(11-9-17-38)41-36(52)53-37(5,6)7/h13-16,22-23,26-29,31,39,43,45H,8-12,17-21,38H2,1-7H3,(H,40,48)(H,41,52)(H,42,47)(H,50,51)/t23-,26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Neurotensin receptor binding affinity by displacement of [3H]- neurotensin |
Bioorg Med Chem Lett 3: 1035-1040 (1993)
Article DOI: 10.1016/S0960-894X(00)80282-6
BindingDB Entry DOI: 10.7270/Q27S7NQN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50019423
(CHEMBL3290105)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(F)cc1)C(=O)NC1(CCCCC1)C(O)=O |(18.77,-17.38,;19.54,-16.04,;21.08,-16.04,;21.85,-17.38,;23.39,-17.38,;24.16,-16.04,;23.39,-14.71,;24.16,-13.38,;25.7,-13.38,;21.85,-14.71,;20.85,-12.98,;21.48,-11.57,;20.33,-10.54,;19,-11.31,;19.32,-12.81,;18.29,-13.96,;18.76,-15.42,;17.73,-16.57,;16.23,-16.25,;15.2,-17.39,;15.75,-14.78,;16.78,-13.64,;20.49,-9.01,;21.9,-8.38,;19.25,-8.1,;19.41,-6.57,;20.9,-7,;21.99,-5.92,;21.62,-4.43,;20.13,-4,;19.04,-5.09,;17.87,-6.68,;17.01,-5.4,;17.2,-8.06,)| Show InChI InChI=1S/C25H26FN3O5/c1-33-20-7-6-8-21(34-2)22(20)19-15-18(28-29(19)17-11-9-16(26)10-12-17)23(30)27-25(24(31)32)13-4-3-5-14-25/h6-12,15H,3-5,13-14H2,1-2H3,(H,27,30)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Displacement of [I125]neurotensin from rat NTS1 overexpressed in CHO-k1 cells by competitive binding Assay |
Bioorg Med Chem Lett 25: 2060-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.083
BindingDB Entry DOI: 10.7270/Q2BZ67RD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281813
((S)-2-((2S,3S)-2-{N'-[(S)-3-[((S)-1-{(S)-3-[(Adama...)Show SMILES CC[C@H](C)[C@H](NNCC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1NCC(=O)[C@H](CCCCN)NC(=O)C12C[C@H]3C[C@H](C[C@H](C3)C1)C2)C(=O)N[C@@H](CC(C)C)C(O)=O |TLB:47:42:49:48.46.45,47:46:49:41.42.43| Show InChI InChI=1S/C45H72N8O8/c1-5-28(4)40(42(58)50-36(43(59)60)17-27(2)3)52-47-25-38(55)35(21-29-11-13-33(54)14-12-29)49-41(57)37-10-8-16-53(37)48-26-39(56)34(9-6-7-15-46)51-44(61)45-22-30-18-31(23-45)20-32(19-30)24-45/h11-14,27-28,30-32,34-37,40,47-48,52,54H,5-10,15-26,46H2,1-4H3,(H,49,57)(H,50,58)(H,51,61)(H,59,60)/t28-,30-,31+,32-,34-,35-,36-,37-,40-,45?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Neurotensin receptor binding affinity by displacement of [3H]- neurotensin |
Bioorg Med Chem Lett 3: 1035-1040 (1993)
Article DOI: 10.1016/S0960-894X(00)80282-6
BindingDB Entry DOI: 10.7270/Q27S7NQN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281805
((S)-2-((2S,3S)-2-{N'-[(S)-3-[((S)-1-{(S)-2-[(Adama...)Show SMILES CC[C@H](C)[C@H](NNCC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC(=O)C12C[C@H]3C[C@H](C[C@H](C3)C1)C2)C(=O)N[C@@H](CC(C)C)C(O)=O |TLB:41:42:46:39.40.45,THB:41:40:46:47.42.43| Show InChI InChI=1S/C44H69N7O8/c1-5-27(4)38(40(55)48-35(42(57)58)17-26(2)3)50-46-25-37(53)34(21-28-11-13-32(52)14-12-28)47-39(54)36-10-8-16-51(36)41(56)33(9-6-7-15-45)49-43(59)44-22-29-18-30(23-44)20-31(19-29)24-44/h11-14,26-27,29-31,33-36,38,46,50,52H,5-10,15-25,45H2,1-4H3,(H,47,54)(H,48,55)(H,49,59)(H,57,58)/t27-,29-,30+,31-,33-,34-,35-,36-,38-,44?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Neurotensin receptor binding affinity by displacement of [3H]- neurotensin |
Bioorg Med Chem Lett 3: 1035-1040 (1993)
Article DOI: 10.1016/S0960-894X(00)80282-6
BindingDB Entry DOI: 10.7270/Q27S7NQN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50069089
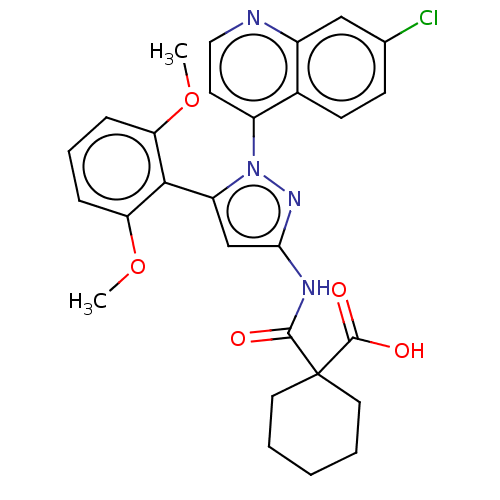
(CHEMBL3403505)Show SMILES COc1cccc(OC)c1-c1cc(NC(=O)C2(CCCCC2)C(O)=O)nn1-c1ccnc2cc(Cl)ccc12 |(-5.51,-6.97,;-4.66,-6.08,;-5.09,-4.6,;-6.59,-4.24,;-7.02,-2.76,;-5.96,-1.65,;-4.47,-2.01,;-3.4,-.89,;-3.75,.29,;-4.03,-3.48,;-2.57,-3.96,;-2.09,-5.43,;-.55,-5.42,;.36,-6.66,;1.89,-6.5,;2.39,-5.37,;2.8,-7.74,;3.73,-8.94,;3.11,-10.35,;1.58,-10.52,;.67,-9.28,;1.29,-7.87,;4.33,-7.57,;5.06,-8.57,;4.83,-6.44,;-.08,-3.96,;-1.33,-3.08,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.73,1.38,;2.66,-.77,;1.33,-1.54,;,-.77,)| Show InChI InChI=1S/C28H27ClN4O5/c1-37-22-7-6-8-23(38-2)25(22)21-16-24(31-26(34)28(27(35)36)12-4-3-5-13-28)32-33(21)20-11-14-30-19-15-17(29)9-10-18(19)20/h6-11,14-16H,3-5,12-13H2,1-2H3,(H,35,36)(H,31,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Displacement of [I125]neurotensin from rat NTS1 overexpressed in CHO-k1 cells by competitive binding Assay |
Bioorg Med Chem Lett 25: 2060-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.083
BindingDB Entry DOI: 10.7270/Q2BZ67RD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366312
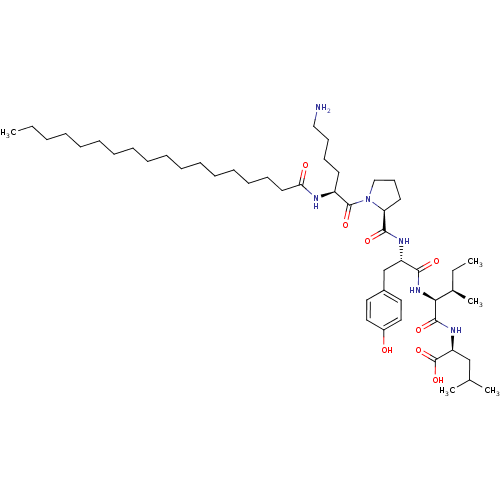
(CHEMBL1790476)Show SMILES CCCCCCCCCCCCCCCCCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C50H86N6O8/c1-6-8-9-10-11-12-13-14-15-16-17-18-19-20-21-27-44(58)52-40(25-22-23-32-51)49(62)56-33-24-26-43(56)47(60)53-41(35-38-28-30-39(57)31-29-38)46(59)55-45(37(5)7-2)48(61)54-42(50(63)64)34-36(3)4/h28-31,36-37,40-43,45,57H,6-27,32-35,51H2,1-5H3,(H,52,58)(H,53,60)(H,54,61)(H,55,59)(H,63,64)/t37-,40+,41+,42+,43+,45+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against rat neurotensin receptor. |
Bioorg Med Chem Lett 3: 2055-2060 (1993)
Article DOI: 10.1016/S0960-894X(01)81014-3
BindingDB Entry DOI: 10.7270/Q2M045XW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50069088
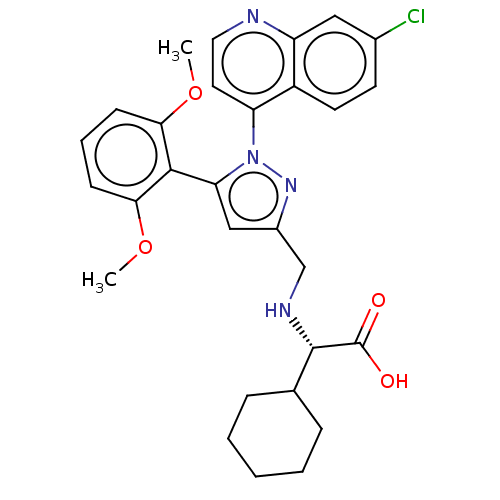
(CHEMBL3403504)Show SMILES COc1cccc(OC)c1-c1cc(CN[C@@H](C2CCCCC2)C(O)=O)nn1-c1ccnc2cc(Cl)ccc12 |r,wU:15.16,(-5.51,-6.97,;-4.66,-6.08,;-5.09,-4.6,;-6.59,-4.24,;-7.02,-2.76,;-5.96,-1.65,;-4.47,-2.01,;-3.4,-.89,;-3.75,.29,;-4.03,-3.48,;-2.57,-3.96,;-2.09,-5.43,;-.55,-5.42,;.36,-6.66,;1.89,-6.5,;2.8,-7.74,;4.33,-7.57,;5.25,-8.81,;6.78,-8.64,;7.4,-7.23,;6.48,-5.99,;4.95,-6.16,;2.18,-9.15,;.96,-9.29,;2.91,-10.14,;-.08,-3.96,;-1.33,-3.08,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.73,1.38,;2.66,-.77,;1.33,-1.54,;,-.77,)| Show InChI InChI=1S/C29H31ClN4O4/c1-37-25-9-6-10-26(38-2)27(25)24-16-20(17-32-28(29(35)36)18-7-4-3-5-8-18)33-34(24)23-13-14-31-22-15-19(30)11-12-21(22)23/h6,9-16,18,28,32H,3-5,7-8,17H2,1-2H3,(H,35,36)/t28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Displacement of [I125]neurotensin from rat NTS1 overexpressed in CHO-k1 cells by competitive binding Assay |
Bioorg Med Chem Lett 25: 2060-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.083
BindingDB Entry DOI: 10.7270/Q2BZ67RD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50366372
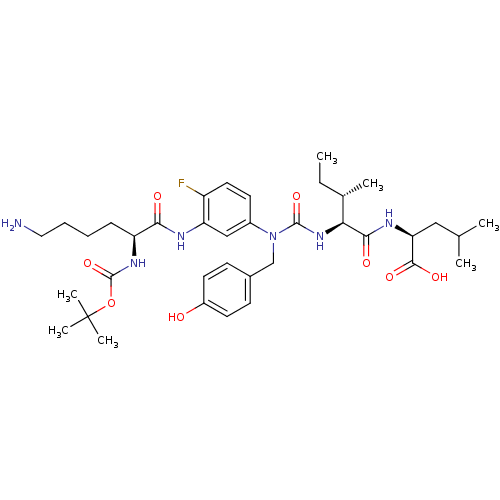
(CHEMBL1793998)Show SMILES CC[C@H](C)[C@H](NC(=O)N(Cc1ccc(O)cc1)c1ccc(F)c(NC(=O)[C@H](CCCCN)NC(=O)OC(C)(C)C)c1)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C37H55FN6O8/c1-8-23(4)31(33(47)41-30(34(48)49)19-22(2)3)43-35(50)44(21-24-12-15-26(45)16-13-24)25-14-17-27(38)29(20-25)40-32(46)28(11-9-10-18-39)42-36(51)52-37(5,6)7/h12-17,20,22-23,28,30-31,45H,8-11,18-19,21,39H2,1-7H3,(H,40,46)(H,41,47)(H,42,51)(H,43,50)(H,48,49)/t23-,28-,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for binding affinity against Neurotensin receptor |
Bioorg Med Chem Lett 5: 1203-1206 (1995)
Article DOI: 10.1016/0960-894X(95)00195-Y
BindingDB Entry DOI: 10.7270/Q2RF5VHW |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50281808
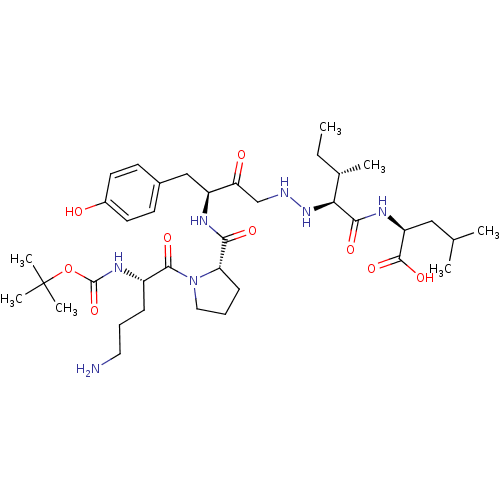
((S)-2-((2S,3S)-2-{N'-[(S)-3-{[(S)-1-((S)-5-Amino-2...)Show SMILES CC[C@H](C)[C@H](NNCC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C37H61N7O9/c1-8-23(4)31(33(48)41-28(35(50)51)19-22(2)3)43-39-21-30(46)27(20-24-13-15-25(45)16-14-24)40-32(47)29-12-10-18-44(29)34(49)26(11-9-17-38)42-36(52)53-37(5,6)7/h13-16,22-23,26-29,31,39,43,45H,8-12,17-21,38H2,1-7H3,(H,40,47)(H,41,48)(H,42,52)(H,50,51)/t23-,26-,27-,28-,29-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Neurotensin receptor binding affinity by displacement of [3H]- neurotensin |
Bioorg Med Chem Lett 3: 1035-1040 (1993)
Article DOI: 10.1016/S0960-894X(00)80282-6
BindingDB Entry DOI: 10.7270/Q27S7NQN |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50069090
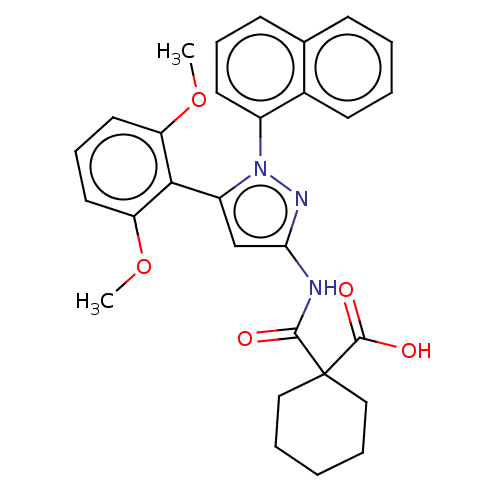
(CHEMBL3403506)Show SMILES COc1cccc(OC)c1-c1cc(NC(=O)C2(CCCCC2)C(O)=O)nn1-c1cccc2ccccc12 |(-5.51,-6.97,;-4.66,-6.08,;-5.09,-4.6,;-6.59,-4.24,;-7.02,-2.76,;-5.96,-1.65,;-4.47,-2.01,;-3.4,-.89,;-3.75,.29,;-4.03,-3.48,;-2.57,-3.96,;-2.09,-5.43,;-.55,-5.42,;.36,-6.66,;1.89,-6.5,;2.39,-5.37,;2.8,-7.74,;3.73,-8.94,;3.11,-10.35,;1.58,-10.52,;.67,-9.28,;1.29,-7.87,;4.33,-7.57,;5.06,-8.57,;4.83,-6.44,;-.08,-3.96,;-1.33,-3.08,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;2.66,-.77,;1.33,-1.54,;,-.77,)| Show InChI InChI=1S/C29H29N3O5/c1-36-23-14-9-15-24(37-2)26(23)22-18-25(30-27(33)29(28(34)35)16-6-3-7-17-29)31-32(22)21-13-8-11-19-10-4-5-12-20(19)21/h4-5,8-15,18H,3,6-7,16-17H2,1-2H3,(H,34,35)(H,30,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Displacement of [I125]neurotensin from rat NTS1 overexpressed in CHO-k1 cells by competitive binding Assay |
Bioorg Med Chem Lett 25: 2060-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.083
BindingDB Entry DOI: 10.7270/Q2BZ67RD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50069091
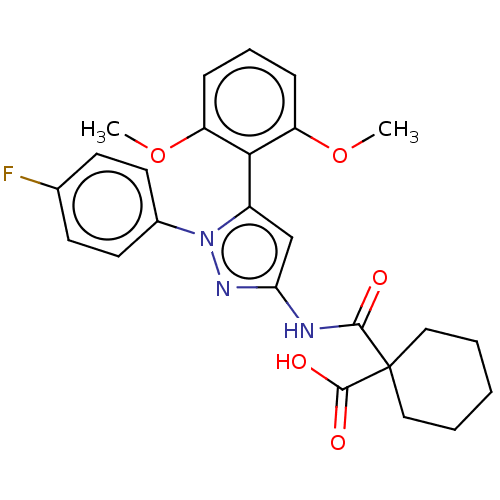
(CHEMBL3403507)Show SMILES COc1cccc(OC)c1-c1cc(NC(=O)C2(CCCCC2)C(O)=O)nn1-c1ccc(F)cc1 |(2.94,7.42,;3.73,6.48,;5.25,6.75,;5.77,8.2,;7.28,8.47,;8.28,7.3,;7.76,5.85,;8.75,4.67,;9.97,4.89,;6.25,5.58,;5.62,4.17,;4.11,3.85,;3.97,2.33,;2.63,1.56,;2.63,.02,;3.7,-.59,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.3,-2.29,;.23,-2.91,;2.36,-2.91,;5.35,1.69,;6.38,2.84,;7.92,2.67,;8.91,3.84,;10.43,3.56,;10.94,2.11,;12.15,1.89,;9.94,.94,;8.43,1.22,)| Show InChI InChI=1S/C25H26FN3O5/c1-33-19-7-6-8-20(34-2)22(19)18-15-21(28-29(18)17-11-9-16(26)10-12-17)27-23(30)25(24(31)32)13-4-3-5-14-25/h6-12,15H,3-5,13-14H2,1-2H3,(H,31,32)(H,27,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Displacement of [I125]neurotensin from rat NTS1 overexpressed in CHO-k1 cells by competitive binding Assay |
Bioorg Med Chem Lett 25: 2060-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.083
BindingDB Entry DOI: 10.7270/Q2BZ67RD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50069092

(CHEMBL3403508)Show SMILES COc1ccccc1-c1cc(NC(=O)C2(CCCCC2)C(O)=O)nn1-c1ccc(F)cc1 Show InChI InChI=1S/C24H24FN3O4/c1-32-20-8-4-3-7-18(20)19-15-21(27-28(19)17-11-9-16(25)10-12-17)26-22(29)24(23(30)31)13-5-2-6-14-24/h3-4,7-12,15H,2,5-6,13-14H2,1H3,(H,30,31)(H,26,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Displacement of [I125]neurotensin from rat NTS1 overexpressed in CHO-k1 cells by competitive binding Assay |
Bioorg Med Chem Lett 25: 2060-4 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.083
BindingDB Entry DOI: 10.7270/Q2BZ67RD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data