

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50163636 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of rat eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50163634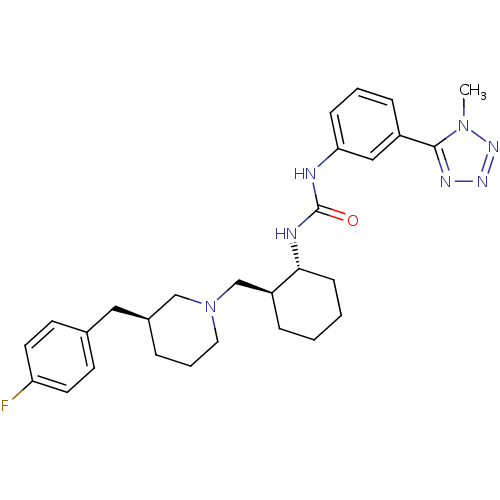 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of rat eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||