

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-xylosidase (Thermoanaerobacter saccharolyticum) | BDBM50182802 (5-(dimethylamino)-N-(6-((3R,4r,5S)-3,4,5-trihydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Thermoanaerobacterium saccharolyticum beta-xylosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-xylosidase (Thermoanaerobacter saccharolyticum) | BDBM50182798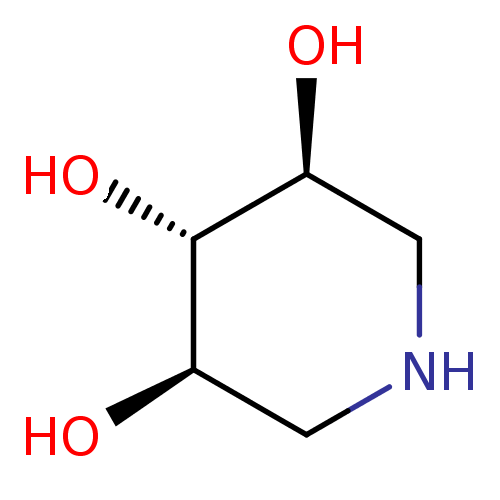 ((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz Curated by ChEMBL | Assay Description Inhibition of Thermoanaerobacterium saccharolyticum beta-xylosidase | Bioorg Med Chem Lett 16: 2067-70 (2006) Article DOI: 10.1016/j.bmcl.2006.01.095 BindingDB Entry DOI: 10.7270/Q26Q1WV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Exo-1,4-beta-xylosidase xlnD (Aspergillus niger) | BDBM50096896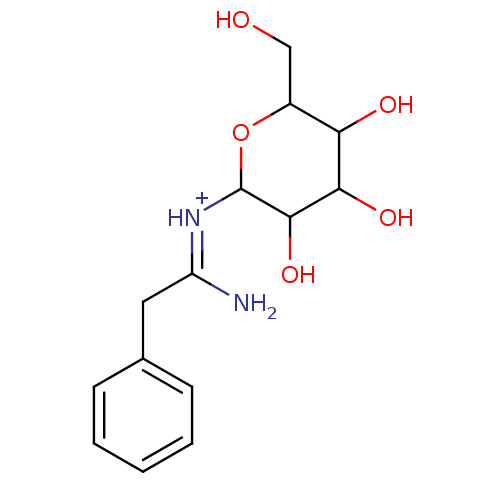 (2-phenyl-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Compound was evaluated for the selective and competitive inhibition of Beta-xylosidase from A. pulverulentus | Bioorg Med Chem Lett 11: 467-70 (2001) BindingDB Entry DOI: 10.7270/Q2R210N1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||