

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518915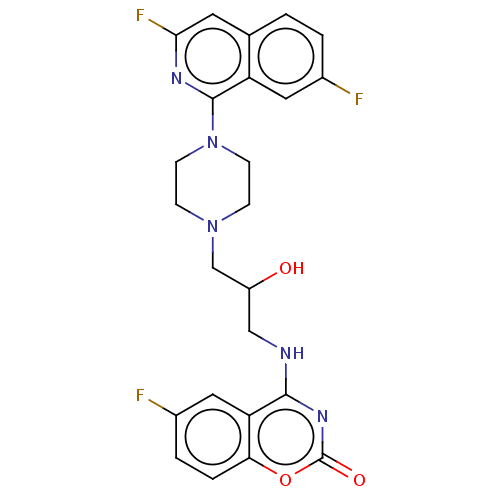 (CHEMBL4583368) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518940 (CHEMBL4541988) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518948 (CHEMBL4574863) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178928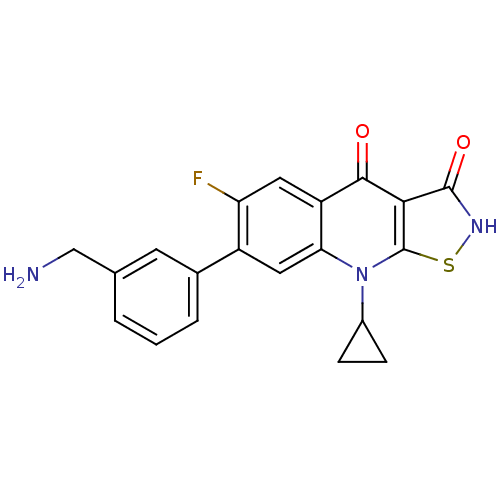 (7-(3-(aminomethyl)phenyl)-9-cyclopropyl-6-fluorois...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178917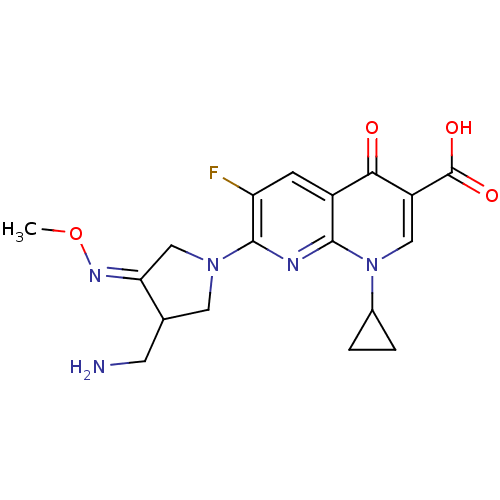 ((S,Z)-7-(3-(aminomethyl)-4-(methoxyimino)pyrrolidi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518924 (CHEMBL4533152) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518911 (CHEMBL4530328) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178926 (9-cyclopropyl-6-fluoro-7-(piperazin-1-yl)isothiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178912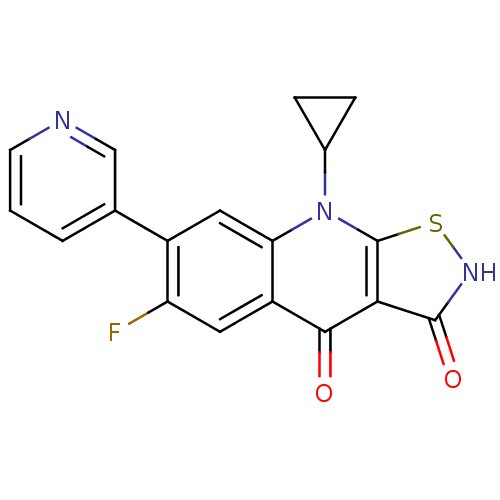 (9-cyclopropyl-6-fluoro-7-(pyridin-3-yl)isothiazolo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178913 (7-(6-(aminomethyl)pyridin-3-yl)-9-cyclopropyl-6-fl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50131428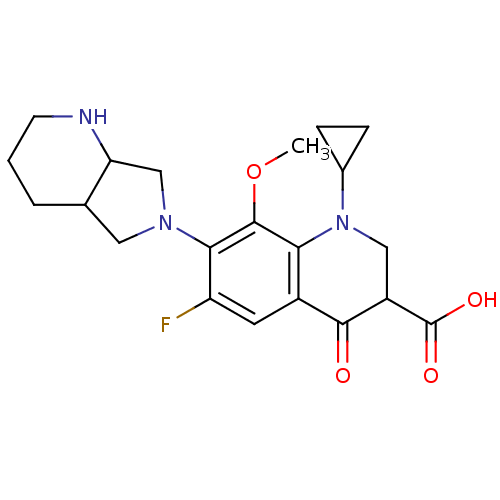 (1-Cyclopropyl-6-fluoro-8-methoxy-7-(1S,7aS)-octahy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50131428 (1-Cyclopropyl-6-fluoro-8-methoxy-7-(1S,7aS)-octahy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178914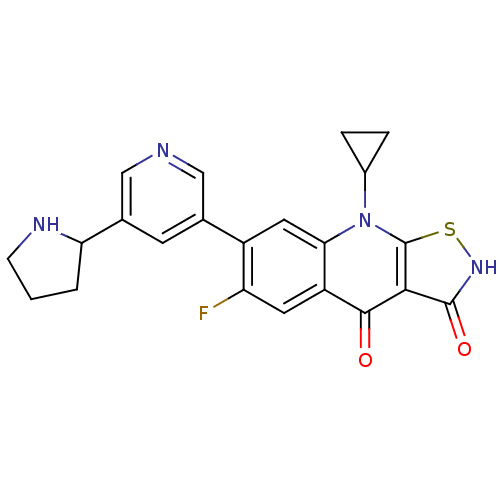 (9-cyclopropyl-6-fluoro-7-(5-(pyrrolidin-2-yl)pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50117914 (1-Cyclopropyl-1,4-dihydro-6-fluoro-8-methoxy-7-(3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178922 (7-(4-(aminomethyl)phenyl)-9-cyclopropyl-6-fluorois...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178924 (9-cyclopropyl-6-fluoro-7-(3-hydroxyphenyl)isothiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518926 (CHEMBL4536608) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518925 (CHEMBL4438807) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518952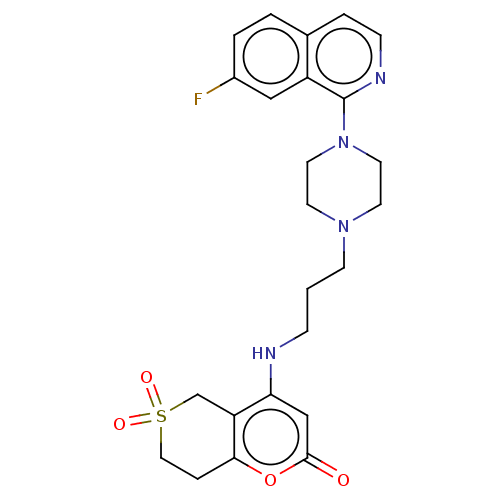 (CHEMBL4476525) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178892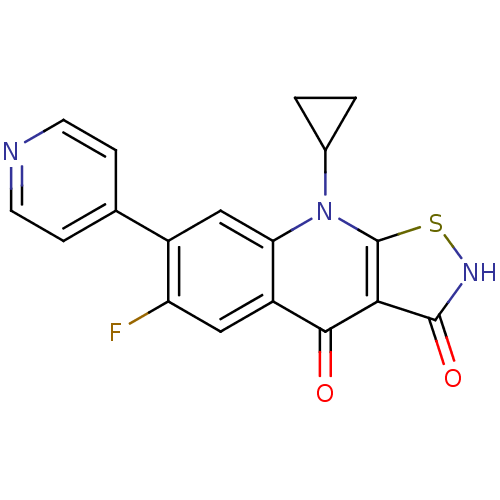 (9-cyclopropyl-6-fluoro-7-(pyridin-4-yl)isothiazolo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of topoisomerase 4 decatenation in Staphylococcus aureus ATCC 29213 | J Med Chem 49: 39-42 (2006) Article DOI: 10.1021/jm051066d BindingDB Entry DOI: 10.7270/Q24B30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178905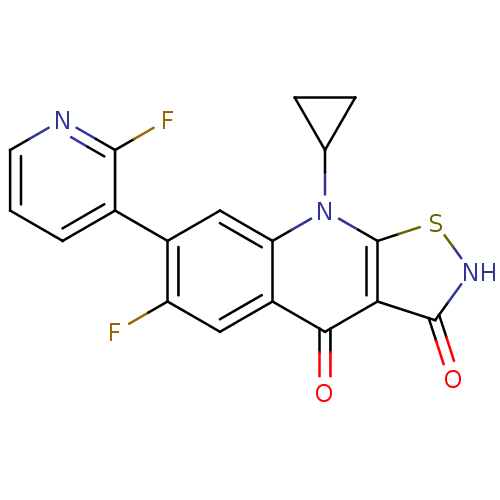 (9-cyclopropyl-6-fluoro-7-(2-fluoropyridin-3-yl)iso...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178929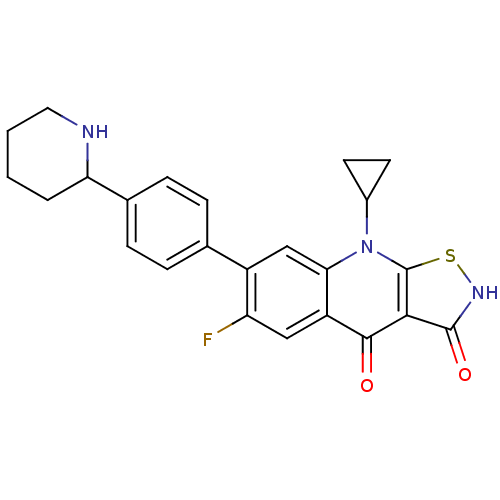 (9-cyclopropyl-6-fluoro-7-(4-(piperidin-2-yl)phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178925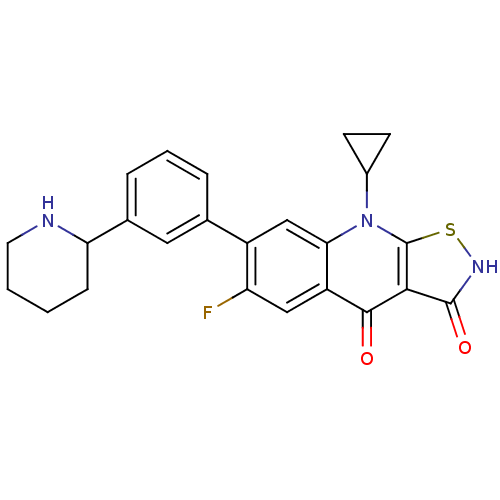 (9-cyclopropyl-6-fluoro-7-(3-(piperidin-2-yl)phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518941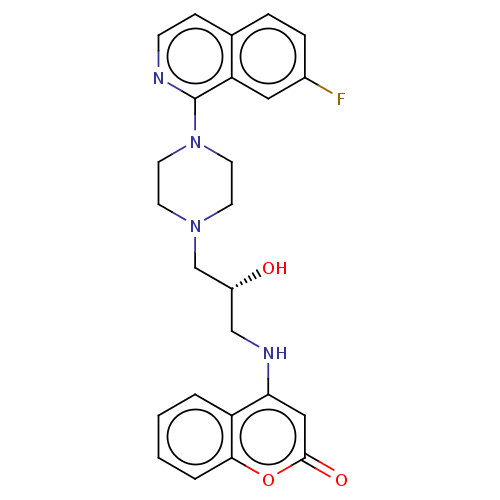 (CHEMBL4465361) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518932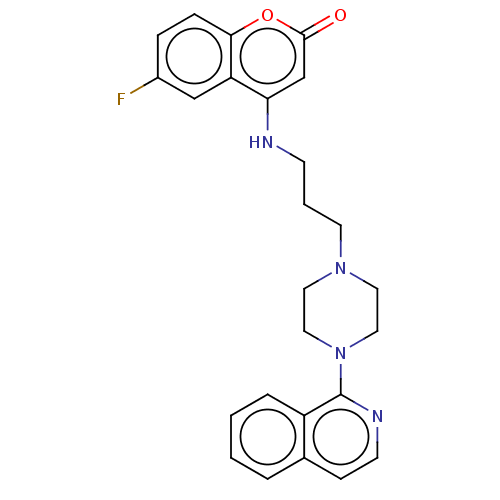 (CHEMBL4467826) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178894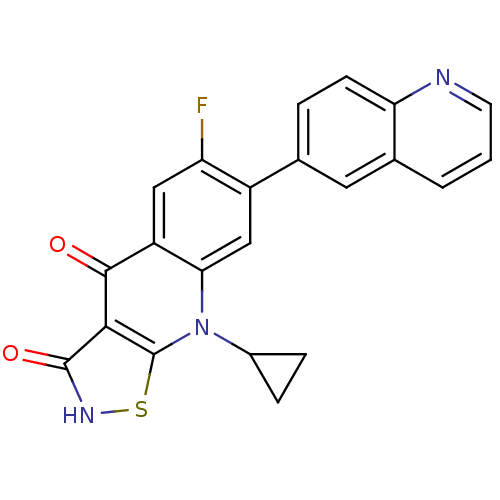 (9-cyclopropyl-6-fluoro-7-(quinolin-6-yl)isothiazol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178897 (9-cyclopropyl-6-fluoro-7-(2-methoxypyrimidin-5-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178904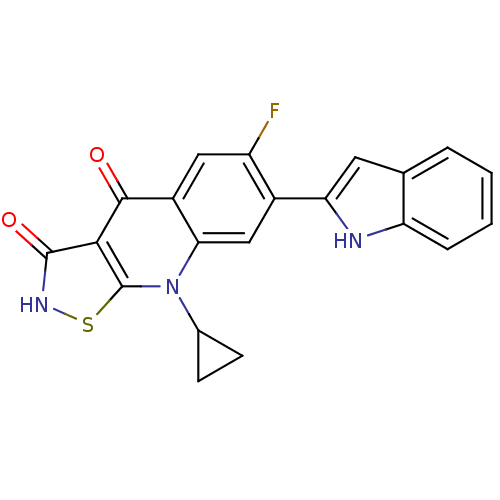 (9-cyclopropyl-6-fluoro-7-(1H-indol-2-yl)isothiazol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178884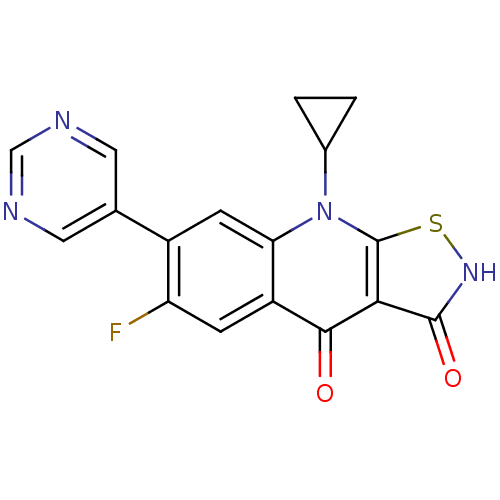 (9-cyclopropyl-6-fluoro-7-(pyrimidin-5-yl)isothiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50045000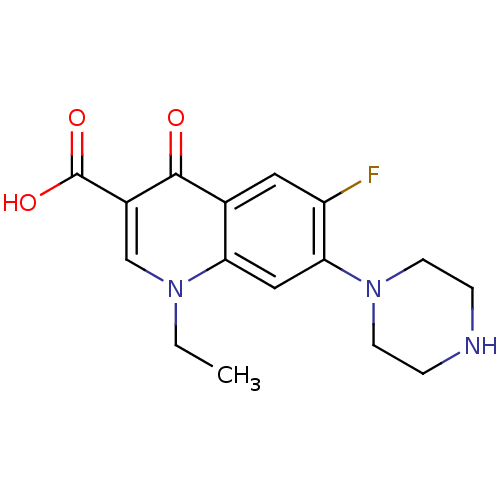 ((NFLX)1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of topoisomerase 4 decatenation in Staphylococcus aureus ATCC 29213 | J Med Chem 49: 39-42 (2006) Article DOI: 10.1021/jm051066d BindingDB Entry DOI: 10.7270/Q24B30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178882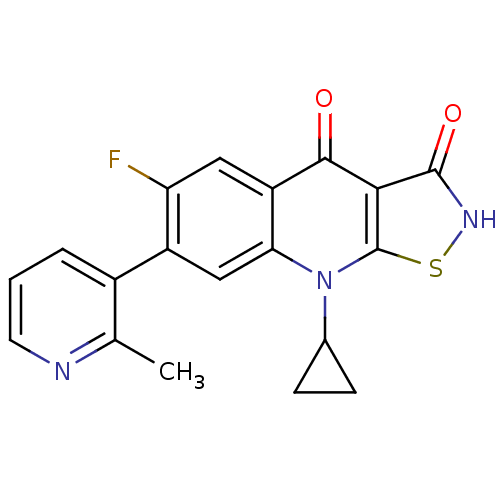 (9-cyclopropyl-6-fluoro-7-(2-methylpyridin-3-yl)iso...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178878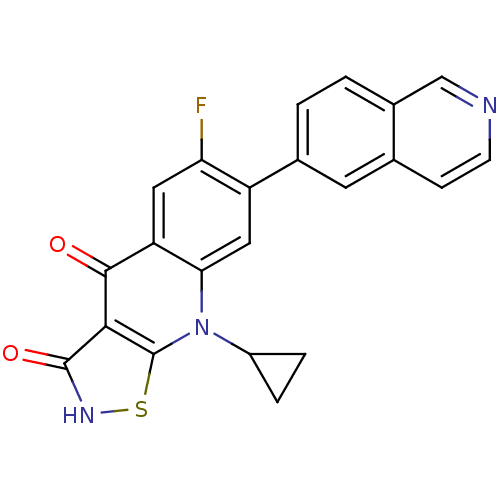 (9-cyclopropyl-6-fluoro-7-(isoquinolin-6-yl)isothia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178909 (9-cyclopropyl-6-fluoro-7-(1-methyl-1H-indol-5-yl)i...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518930 (CHEMBL4453061) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178907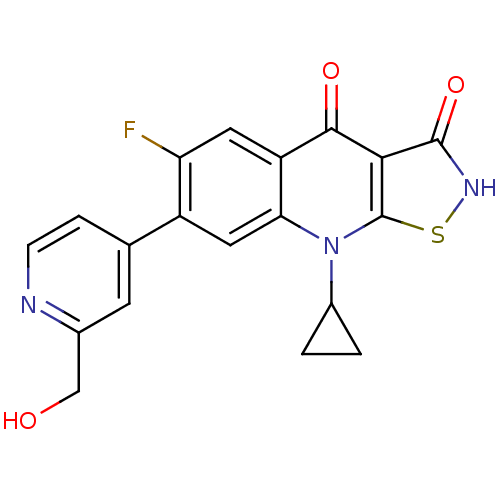 (9-cyclopropyl-6-fluoro-7-(2-(hydroxymethyl)pyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178910 (9-cyclopropyl-6-fluoro-7-(5-(piperidin-2-yl)pyridi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518910 (CHEMBL4554663) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518933 (CHEMBL4567626) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518951 (CHEMBL4570423) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50180009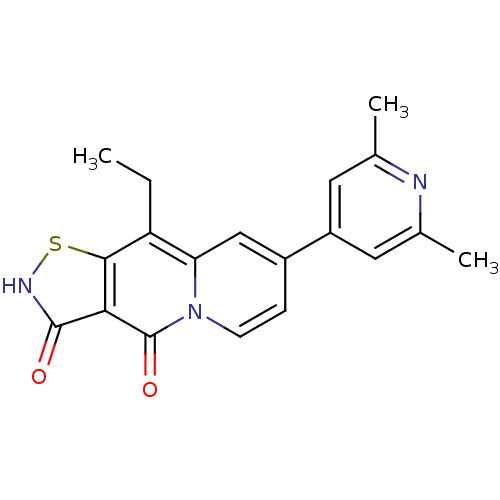 (7-(2,6-dimethylpyridin-4-yl)-9-ethyl-1-thia-2,4a-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of topoisomerase 4 decatenation in Staphylococcus aureus ATCC 29213 | J Med Chem 49: 39-42 (2006) Article DOI: 10.1021/jm051066d BindingDB Entry DOI: 10.7270/Q24B30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178915 (9-cyclopropyl-6-fluoro-7-phenylisothiazolo[5,4-b]q...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518918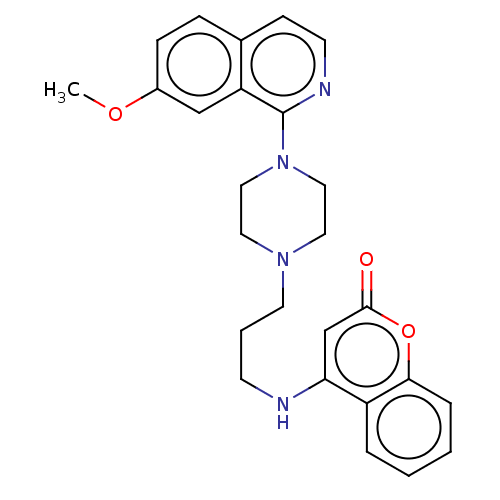 (CHEMBL4569403) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518944 (CHEMBL4537564) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178920 (9-cyclopropyl-6-fluoro-7-(3-fluorophenyl)isothiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against wild type Staphylococcus aureus topoisomerase 4 | Bioorg Med Chem Lett 16: 1272-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.065 BindingDB Entry DOI: 10.7270/Q22R3R76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50518934 (CHEMBL4530941) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini S.p.A. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus ATCC 29213 DNA topoisomerase 4 subunit ParC assessed as reduction in decatenation of kinetoplast DNA incubated fo... | J Med Chem 62: 7445-7472 (2019) Article DOI: 10.1021/acs.jmedchem.9b00394 BindingDB Entry DOI: 10.7270/Q2Z03CKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50178888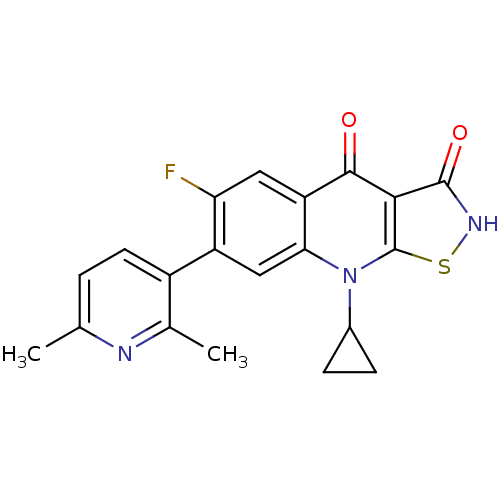 (9-cyclopropyl-7-(2,6-dimethylpyridin-3-yl)-6-fluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Staphylococcus aureus ATCC 29213 wild type Topo 4 | Bioorg Med Chem Lett 16: 1277-81 (2006) Article DOI: 10.1016/j.bmcl.2005.11.064 BindingDB Entry DOI: 10.7270/Q26D5SKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 4 subunit A (Staphylococcus aureus) | BDBM50180011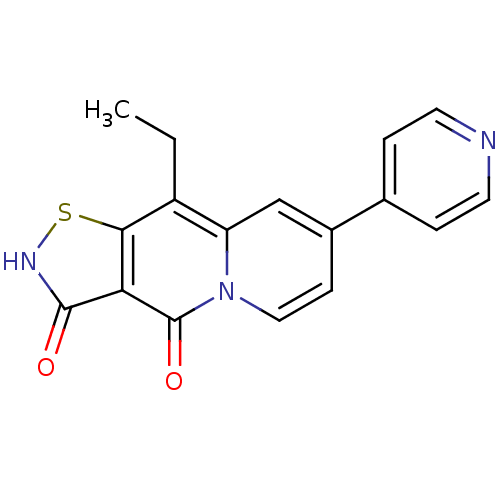 (9-ethyl-7-pyridin-4-yl-1-thia-2,4a-diaza-cyclopent...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of topoisomerase 4 decatenation in Staphylococcus aureus ATCC 29213 | J Med Chem 49: 39-42 (2006) Article DOI: 10.1021/jm051066d BindingDB Entry DOI: 10.7270/Q24B30W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 107 total ) | Next | Last >> |