

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462353 (US10766902, Example 67) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | CHEMBL5278196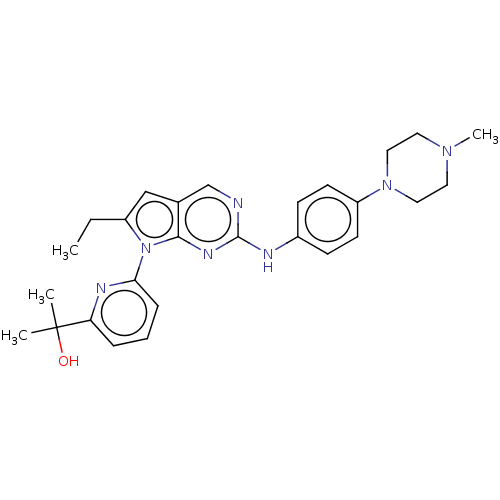 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of angiotensin converting enzyme isolated from rat lung. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462308 (US10766902, Example 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462323 (US10766902, Example 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462376 (US10766902, Example 89) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462377 (US10766902, Example 90) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | CHEMBL5284925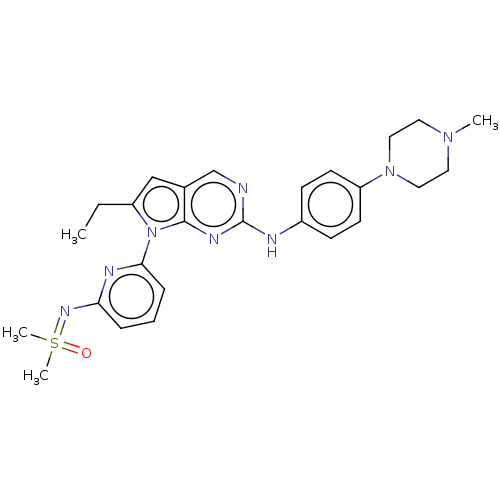 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of angiotensin converting enzyme isolated from rat lung. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462343 (US10766902, Example 57) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462327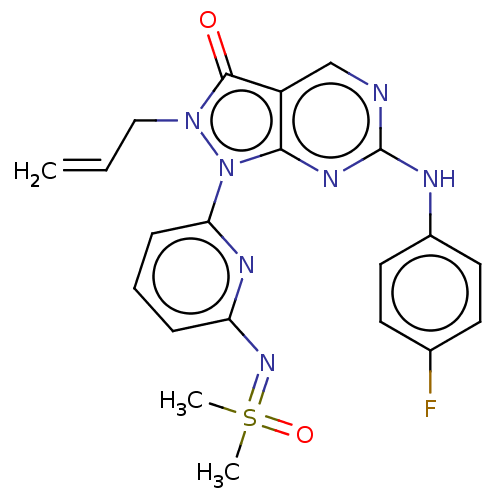 (US10766902, Example 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462360 (US10766902, Example 74) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462340 (US10766902, Example 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462354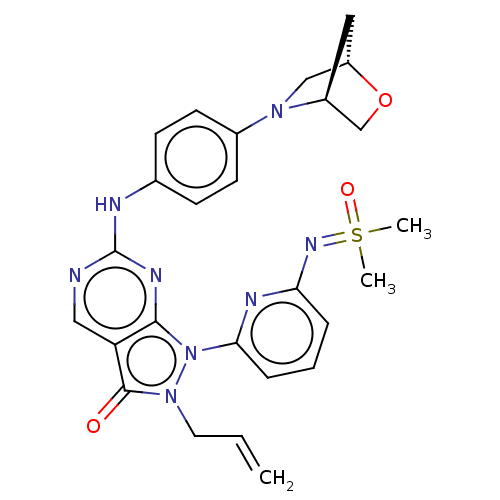 (US10766902, Example 68) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462367 (US10766902, Example 80) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462295 (US10766902, Example 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462348 (US10766902, Example 62) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462355 (US10766902, Example 69) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462375 (US10766902, Example 88) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462344 (US10766902, Example 58) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462313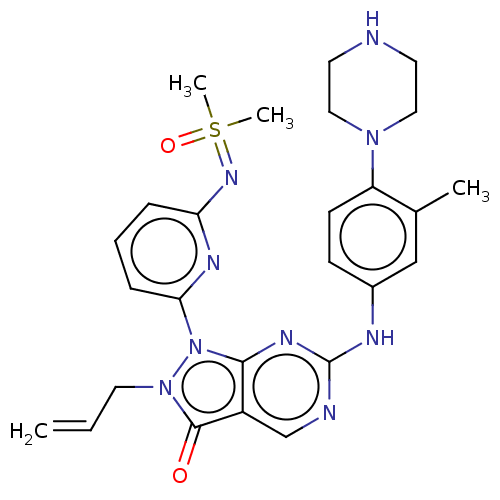 (US10766902, Example 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462380 (US10766902, Example 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462383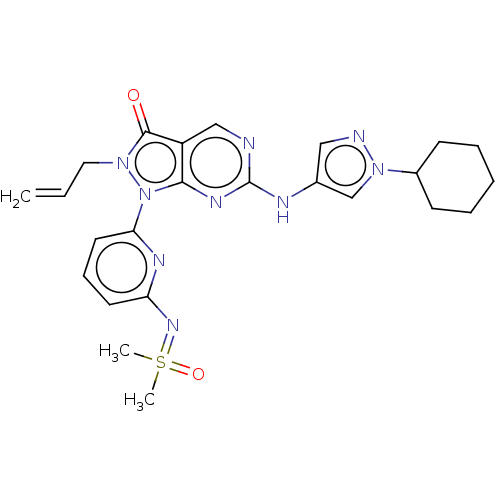 (US10766902, Example 96) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462336 (US10766902, Example 50) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462369 (US10766902, Example 82) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462370 (US10766902, Example 83) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462330 (US10766902, Example 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462373 (US10766902, Example 86) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | CHEMBL5279054 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of Neutral Endopeptidase isolated from rat kidney. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462374 (US10766902, Example 87) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462352 (US10766902, Example 66) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462339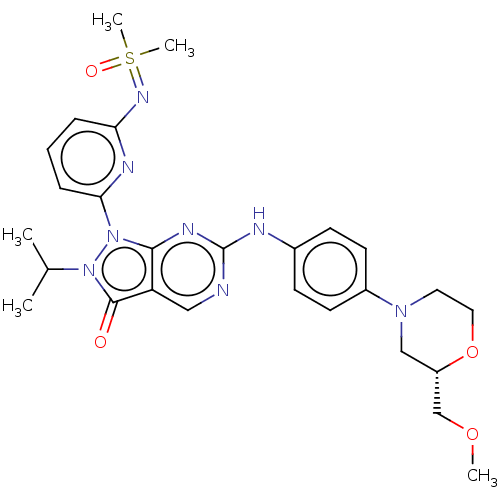 (US10766902, Example 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462332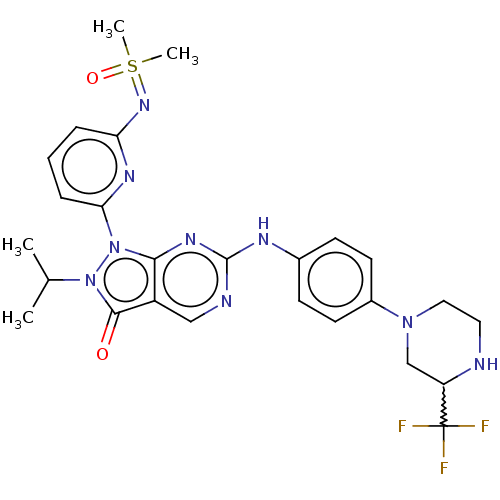 (US10766902, Example 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462319 (US10766902, Example 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462317 (US10766902, Example 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462299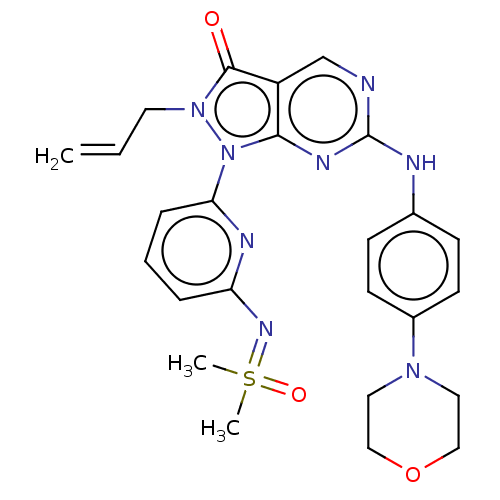 (US10766902, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | CHEMBL5282382 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of Neutral Endopeptidase isolated from rat kidney. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | CHEMBL5269524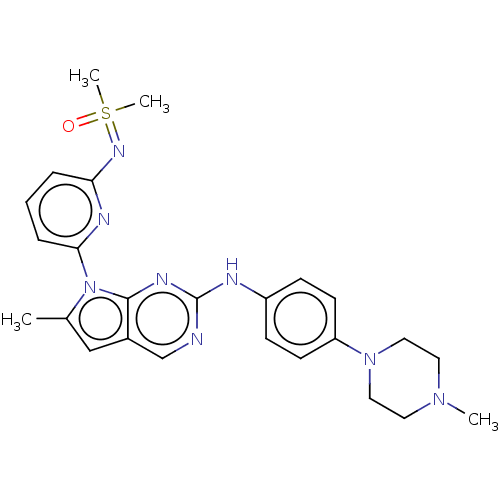 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of angiotensin converting enzyme isolated from rat lung. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM50240826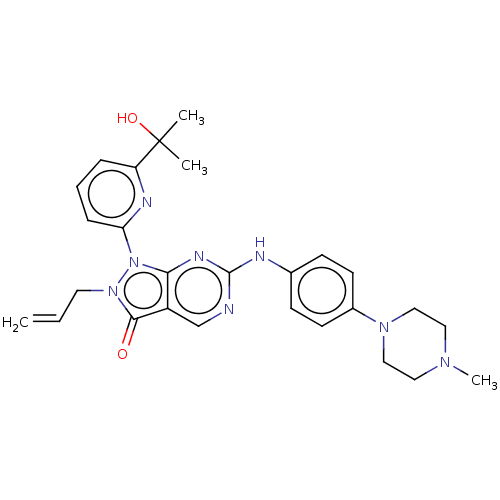 (AZD-1775 | MK-1775 | US11124518, Example AZD1775 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In vitro inhibition of angiotensin converting enzyme isolated from rat lung. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | CHEMBL5284989 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of angiotensin converting enzyme isolated from rat lung. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462363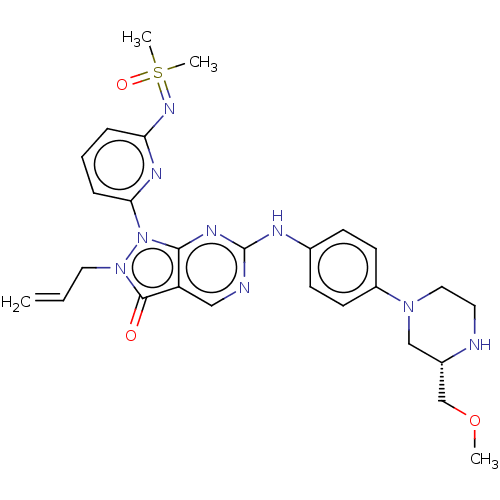 (US10766902, Example 76) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | CHEMBL5281278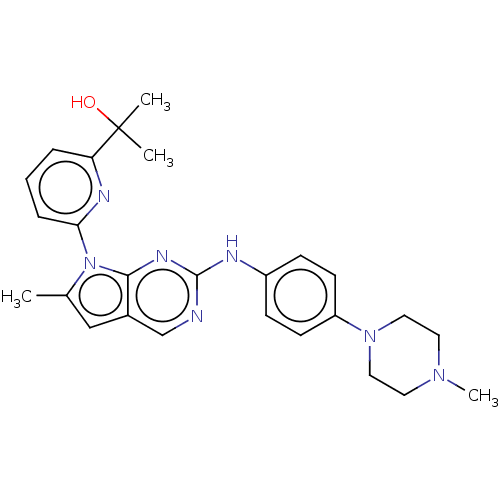 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of angiotensin converting enzyme isolated from rat lung. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462347 (US10766902, Example 61) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462337 (US10766902, Example 51) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462372 (US10766902, Example 85) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462322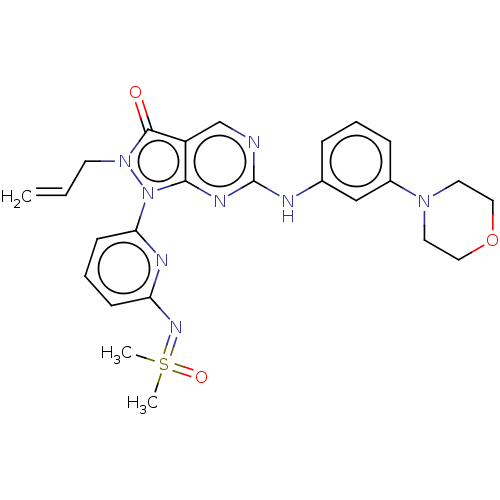 (US10766902, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM549213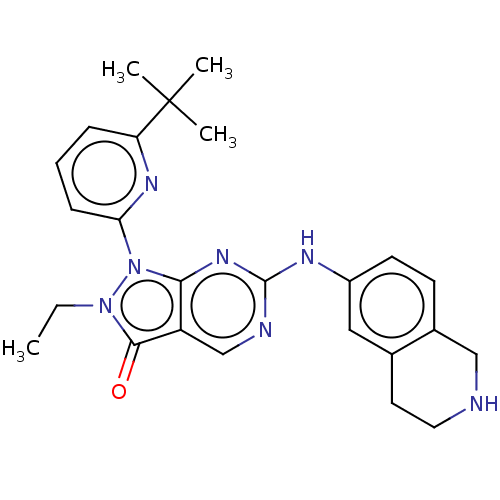 (US11299493, Compound 1.131 | US11299493, S-33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50 values of compounds against WEE1 kinase enzyme were determined by LanthaScreen Terbium Labeled TR-FRET assay. Kinase assays were performed in 1×... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84G37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM549228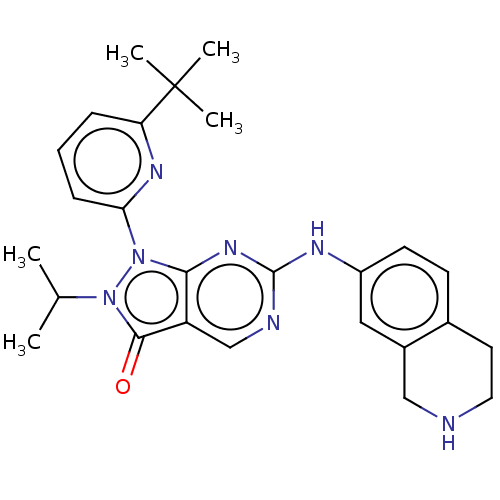 (US11299493, Compound 1.144 | US11299493, S-47) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description IC50 values of compounds against WEE1 kinase enzyme were determined by LanthaScreen Terbium Labeled TR-FRET assay. Kinase assays were performed in 1×... | Citation and Details BindingDB Entry DOI: 10.7270/Q2P84G37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462312 (US10766902, Example 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462292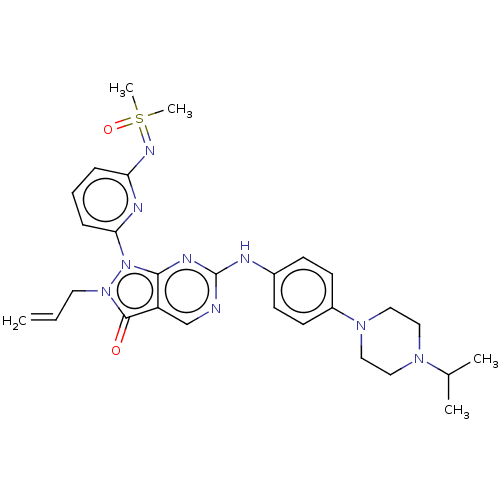 (US10766902, Example 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | BDBM462315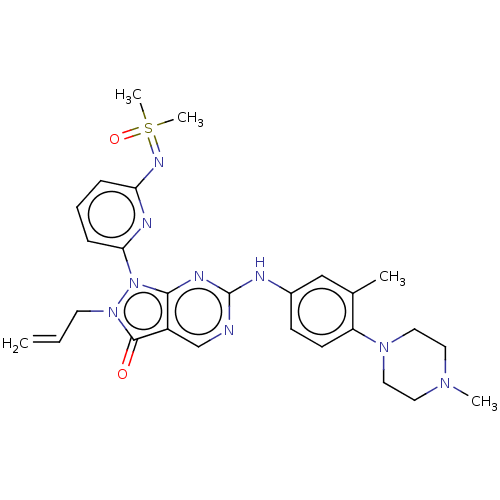 (US10766902, Example 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMAC DISCOVERY LIMITED US Patent | Assay Description All reactions took place in 60 μL volumes in reaction buffer containing 40 mM Tris-HCl and 20 mM magnesium chloride, supplemented with 0.1 mg/mL... | US Patent US10766902 (2020) BindingDB Entry DOI: 10.7270/Q2KD21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Wee1-like protein kinase (Homo sapiens (Human)) | CHEMBL5290439 | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of angiotensin converting enzyme isolated from rat lung. | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1213 total ) | Next | Last >> |