

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (West Nile virus) | BDBM50513893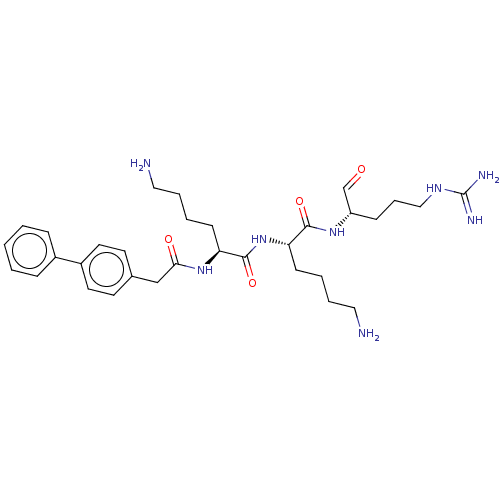 (CHEMBL506092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alagoas Curated by ChEMBL | Assay Description Inhibition of West Nile virus protease | Bioorg Med Chem 27: 3963-3978 (2019) Article DOI: 10.1016/j.bmc.2019.07.038 BindingDB Entry DOI: 10.7270/Q2DN48CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM35491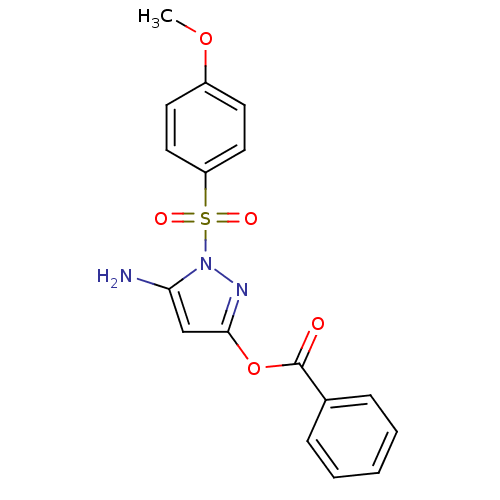 (Benzoic acid 5-amino-1-(4-methoxy-benzenesulfonyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease by fluorescence assay | Bioorg Med Chem Lett 19: 5773-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.150 BindingDB Entry DOI: 10.7270/Q2DZ08DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM35491 (Benzoic acid 5-amino-1-(4-methoxy-benzenesulfonyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Molecular Library Screening Center Curated by PubChem BioAssay | Assay Description The HTS assay to identify Inhibitors of West Nile Virus (WNV) NS2bNS3 Proteinase was proposed by Dr Alex Strongin of the Burnham Institute XO1-MH0776... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2222S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM35491 (Benzoic acid 5-amino-1-(4-methoxy-benzenesulfonyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM35509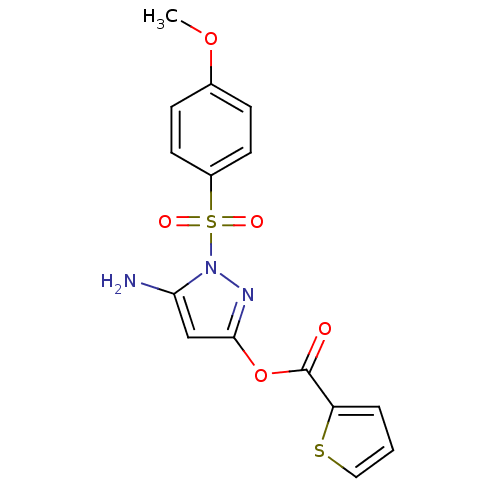 (2-thiophenecarboxylic acid [5-amino-1-(4-methoxyph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Molecular Library Screening Center Curated by PubChem BioAssay | Assay Description The HTS assay to identify Inhibitors of West Nile Virus (WNV) NS2bNS3 Proteinase was proposed by Dr Alex Strongin of the Burnham Institute XO1-MH0776... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2222S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM35509 (2-thiophenecarboxylic acid [5-amino-1-(4-methoxyph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease by fluorescence assay | Bioorg Med Chem Lett 19: 5773-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.150 BindingDB Entry DOI: 10.7270/Q2DZ08DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | CHEMBL5275144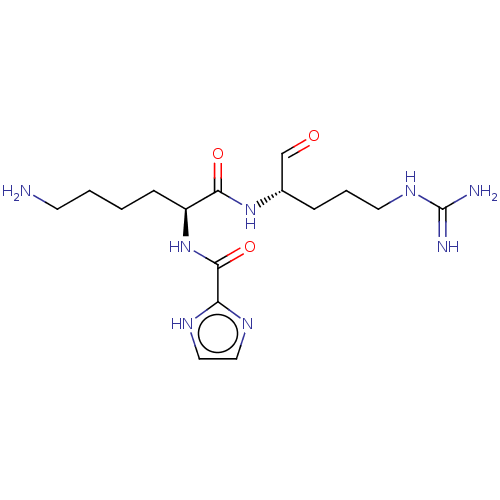 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50567466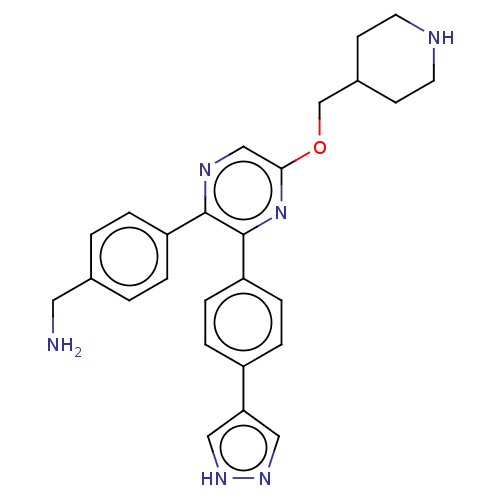 (CHEMBL4862682) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition West Nile virus NS3B/NS3 protease using 7-amino-4-methylcoumarin as substrate by fluorescence assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02070 BindingDB Entry DOI: 10.7270/Q20G3PWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50567466 (CHEMBL4862682) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM39394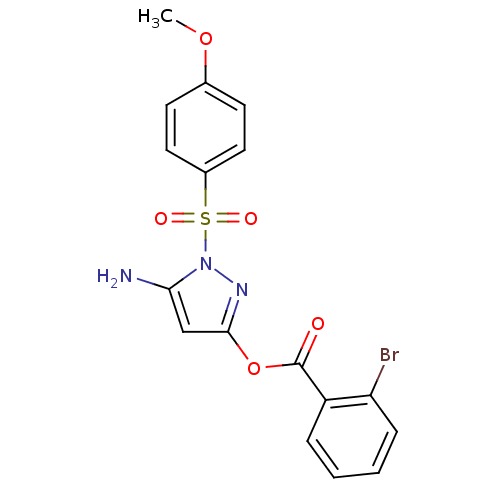 (2-bromobenzoic acid [5-amino-1-(4-methoxyphenyl)su...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 297 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Molecular Library Screening Center Curated by PubChem BioAssay | Assay Description The HTS assay to identify Inhibitors of West Nile Virus (WNV) NS2bNS3 Proteinase was proposed by Dr Alex Strongin of the Burnham Institute XO1-MH0776... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2222S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50302021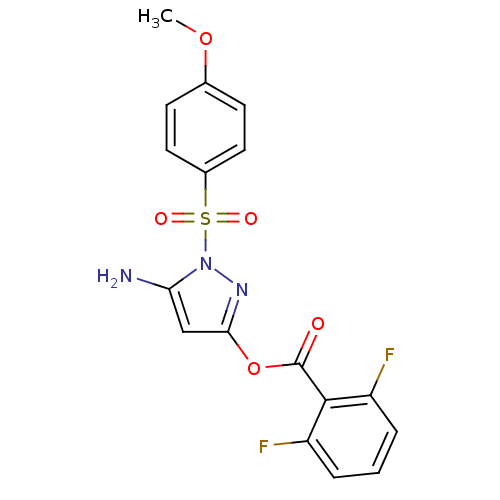 (5-amino-1-(4-methoxyphenylsulfonyl)-1H-pyrazol-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease by fluorescence assay | Bioorg Med Chem Lett 19: 5773-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.150 BindingDB Entry DOI: 10.7270/Q2DZ08DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50030457 (CHEMBL3344314) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of KwaZulu-Natal Curated by ChEMBL | Assay Description Inhibition of West Nile virus NS2B-NS3 protease | Eur J Med Chem 87: 677-702 (2014) Article DOI: 10.1016/j.ejmech.2014.10.010 BindingDB Entry DOI: 10.7270/Q2WW7K9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50030458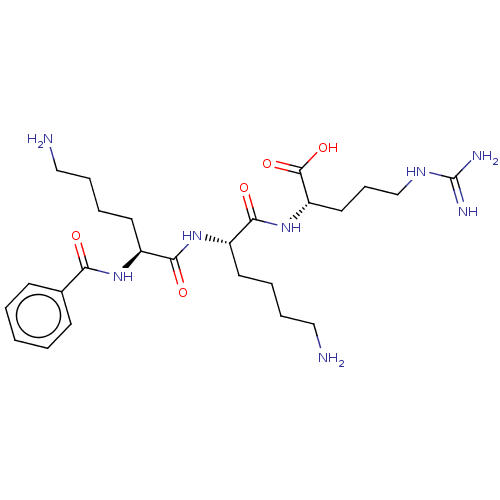 (CHEMBL3344313) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of KwaZulu-Natal Curated by ChEMBL | Assay Description Inhibition of West Nile virus NS2B-NS3 protease | Eur J Med Chem 87: 677-702 (2014) Article DOI: 10.1016/j.ejmech.2014.10.010 BindingDB Entry DOI: 10.7270/Q2WW7K9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | CHEMBL5269219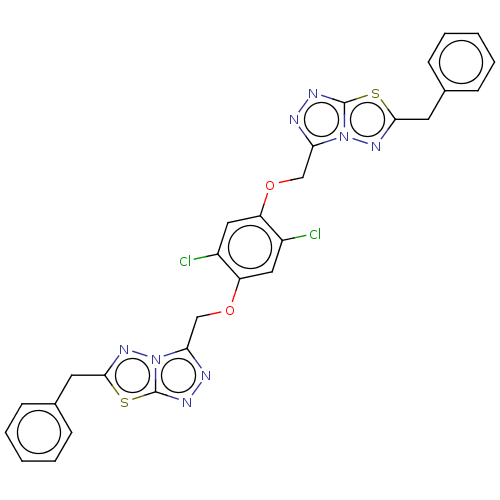 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50587014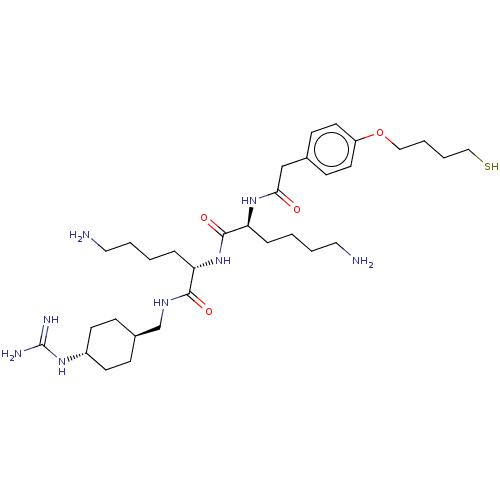 (CHEMBL5094815) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of C-terminal His-tagged WNV NSB2 (52 to 96 amino acids)-NS3 (1 to 184 amino acids) protease expressed in Escherichia coli BL21(DE3) using... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00515 BindingDB Entry DOI: 10.7270/Q21N8511 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50567470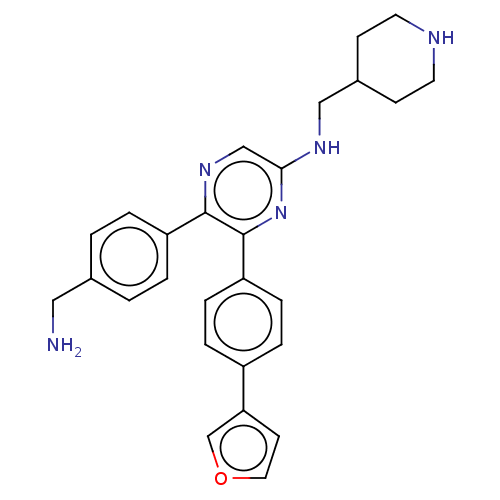 (CHEMBL4862353) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition West Nile virus NS3B/NS3 protease using 7-amino-4-methylcoumarin as substrate by fluorescence assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02070 BindingDB Entry DOI: 10.7270/Q20G3PWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM32761 (2-furancarboxylic acid [5-amino-1-(4-methoxyphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 553 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Molecular Library Screening Center Curated by PubChem BioAssay | Assay Description The HTS assay to identify Inhibitors of West Nile Virus (WNV) NS2bNS3 Proteinase was proposed by Dr Alex Strongin of the Burnham Institute XO1-MH0776... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2222S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50108877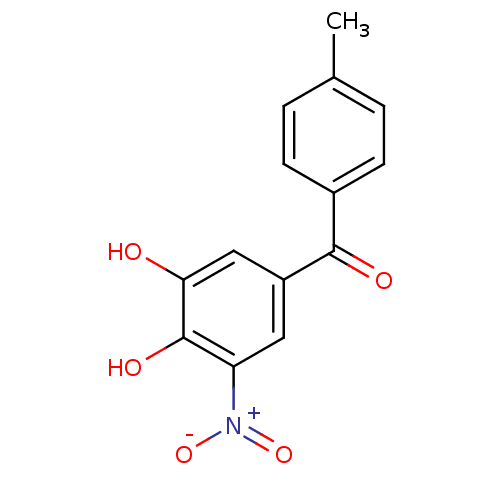 ((3,4-Dihydroxy-5-nitro-phenyl)-p-tolyl-methanone |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109138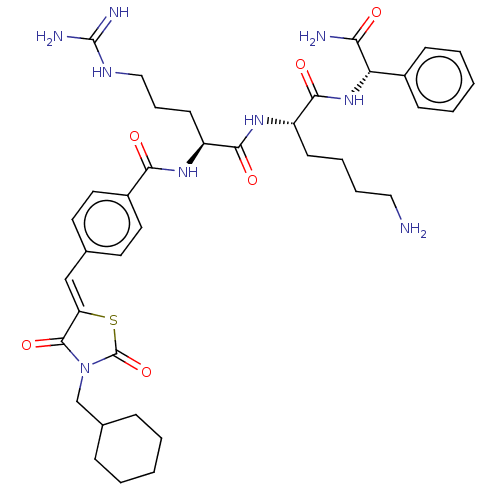 (CHEMBL3601351) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50567467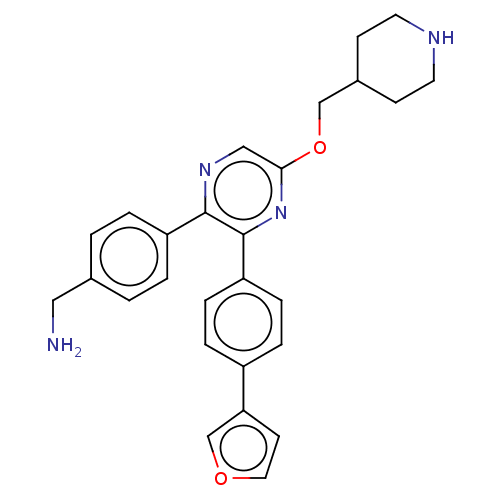 (CHEMBL4850391) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition West Nile virus NS3B/NS3 protease using 7-amino-4-methylcoumarin as substrate by fluorescence assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02070 BindingDB Entry DOI: 10.7270/Q20G3PWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50522790 (CHEMBL3741422) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of West Nile virus NS2B-NS3 protease preincubated with protein for 15 mins followed by Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)-Tyr substrate a... | Bioorg Med Chem Lett 29: 1913-1917 (2019) Article DOI: 10.1016/j.bmcl.2019.05.054 BindingDB Entry DOI: 10.7270/Q2T15723 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50567473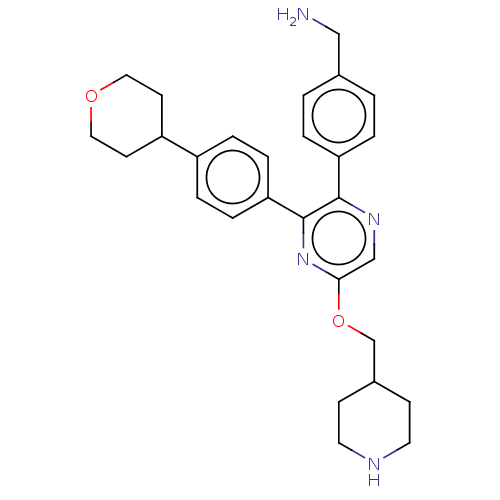 (CHEMBL4872757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition West Nile virus NS3B/NS3 protease using 7-amino-4-methylcoumarin as substrate by fluorescence assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02070 BindingDB Entry DOI: 10.7270/Q20G3PWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50567469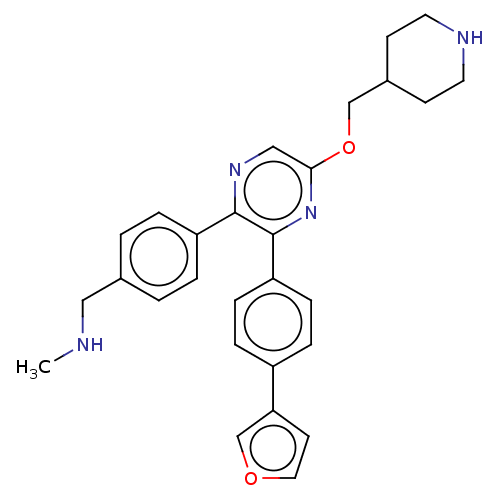 (CHEMBL4856850) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition West Nile virus NS3B/NS3 protease using 7-amino-4-methylcoumarin as substrate by fluorescence assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02070 BindingDB Entry DOI: 10.7270/Q20G3PWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50567468 (CHEMBL4853830) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition West Nile virus NS3B/NS3 protease using 7-amino-4-methylcoumarin as substrate by fluorescence assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02070 BindingDB Entry DOI: 10.7270/Q20G3PWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50302022 (5-amino-1-(4-methoxyphenylsulfonyl)-1H-pyrazol-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease by fluorescence assay | Bioorg Med Chem Lett 19: 5773-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.150 BindingDB Entry DOI: 10.7270/Q2DZ08DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109050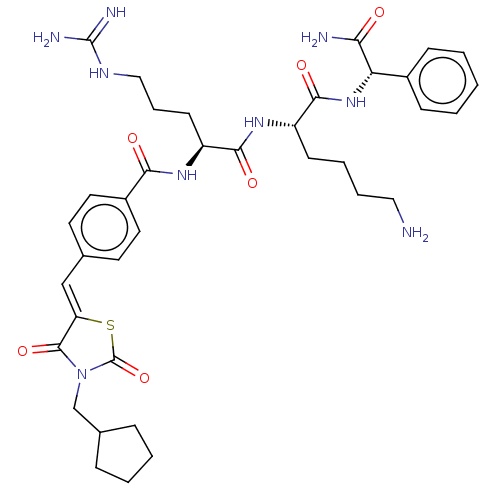 (CHEMBL3601349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109046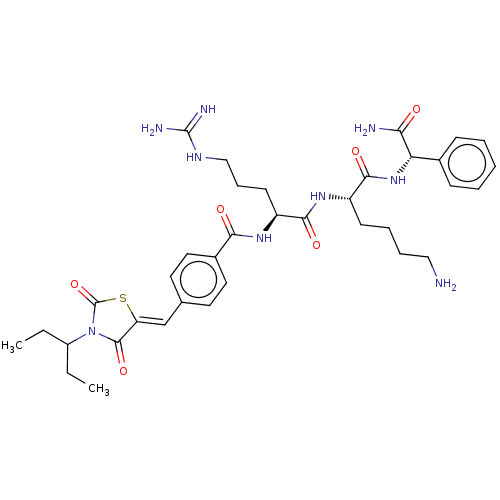 (CHEMBL3601345) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109139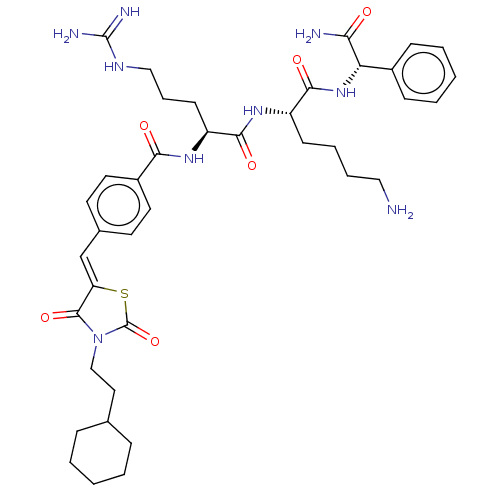 (CHEMBL3601352) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM32783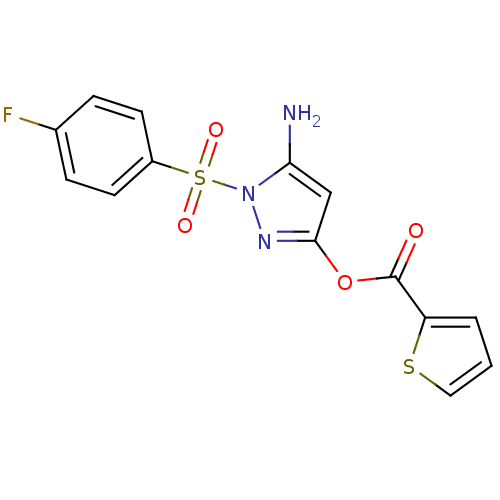 (2-thiophenecarboxylic acid [5-amino-1-(4-fluorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Molecular Library Screening Center Curated by PubChem BioAssay | Assay Description The HTS assay to identify Inhibitors of West Nile Virus (WNV) NS2bNS3 Proteinase was proposed by Dr Alex Strongin of the Burnham Institute XO1-MH0776... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2222S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50542823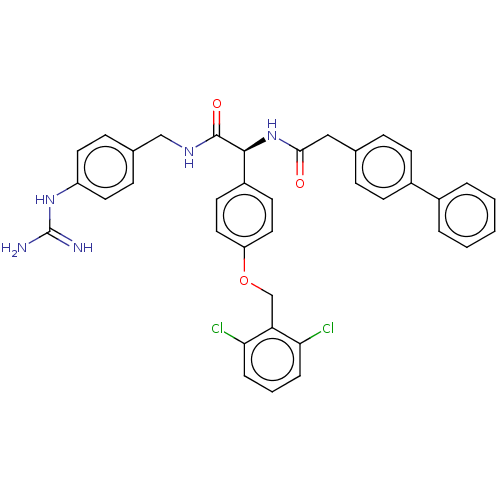 (CHEMBL4633312) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of West Nile virus NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as... | J Med Chem 63: 8179-8197 (2020) Article DOI: 10.1021/acs.jmedchem.0c00413 BindingDB Entry DOI: 10.7270/Q26H4N01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50241035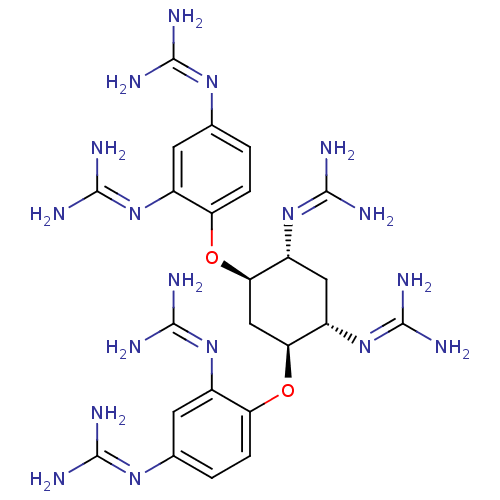 (1-[(1R,2R,4S,5S)-5-carbamimidamido-2,4-bis(2,4-dic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Prague Curated by ChEMBL | Assay Description Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Escherichia coli | Bioorg Med Chem 18: 1434-40 (2010) Article DOI: 10.1016/j.bmc.2010.01.015 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50302023 (5-amino-1-(4-methoxyphenylsulfonyl)-1H-pyrazol-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease by fluorescence assay | Bioorg Med Chem Lett 19: 5773-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.150 BindingDB Entry DOI: 10.7270/Q2DZ08DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM39396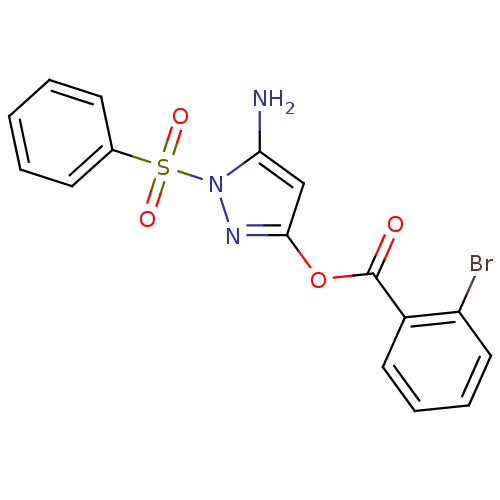 (2-bromobenzoic acid (5-amino-1-besyl-pyrazol-3-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Molecular Library Screening Center Curated by PubChem BioAssay | Assay Description The HTS assay to identify Inhibitors of West Nile Virus (WNV) NS2bNS3 Proteinase was proposed by Dr Alex Strongin of the Burnham Institute XO1-MH0776... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2222S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109142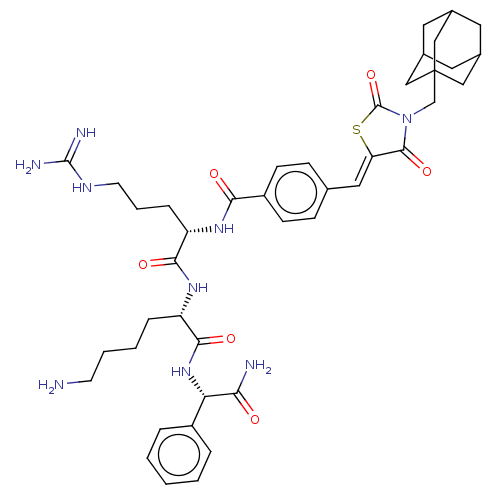 (CHEMBL3601354) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50567471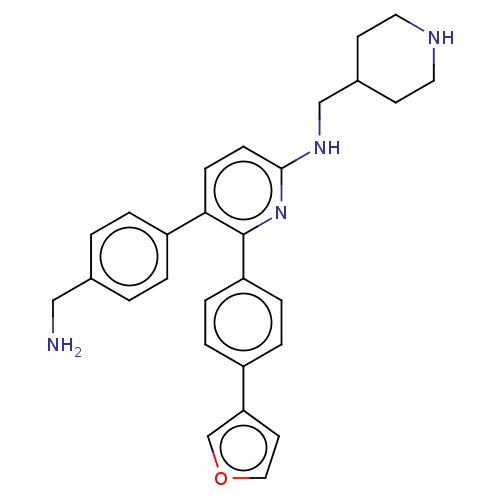 (CHEMBL4876865) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition West Nile virus NS3B/NS3 protease using 7-amino-4-methylcoumarin as substrate by fluorescence assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02070 BindingDB Entry DOI: 10.7270/Q20G3PWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50567472 (CHEMBL4848934) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition West Nile virus NS3B/NS3 protease using 7-amino-4-methylcoumarin as substrate by fluorescence assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02070 BindingDB Entry DOI: 10.7270/Q20G3PWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109047 (CHEMBL3601346) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM32778 (MLS000081703 | SMR000062919 | [2-(2-cyanoethyl)-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Molecular Library Screening Center Curated by PubChem BioAssay | Assay Description The HTS assay to identify Inhibitors of West Nile Virus (WNV) NS2bNS3 Proteinase was proposed by Dr Alex Strongin of the Burnham Institute XO1-MH0776... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2222S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM32778 (MLS000081703 | SMR000062919 | [2-(2-cyanoethyl)-5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease by fluorescence assay | Bioorg Med Chem Lett 19: 5773-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.150 BindingDB Entry DOI: 10.7270/Q2DZ08DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109049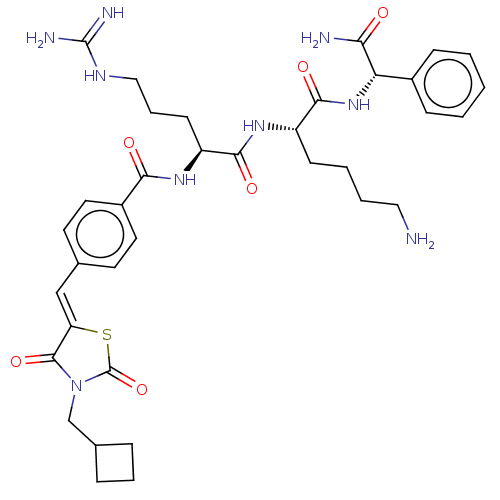 (CHEMBL3601348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50127623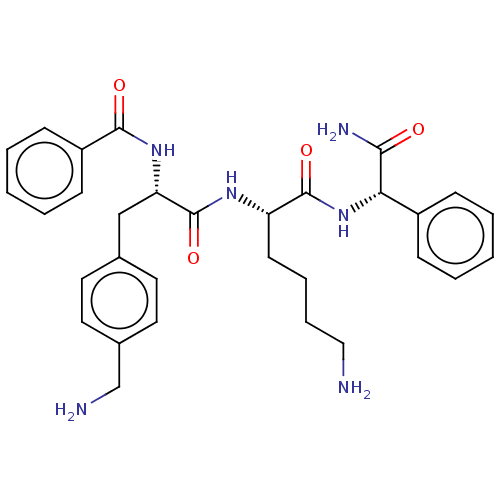 (CHEMBL3628260) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of West Nile virus NS2B-NS3 protease preincubated with protein for 15 mins followed by Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)-Tyr substrate a... | J Med Chem 58: 7719-33 (2015) Article DOI: 10.1021/acs.jmedchem.5b00612 BindingDB Entry DOI: 10.7270/Q2SB47K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM32756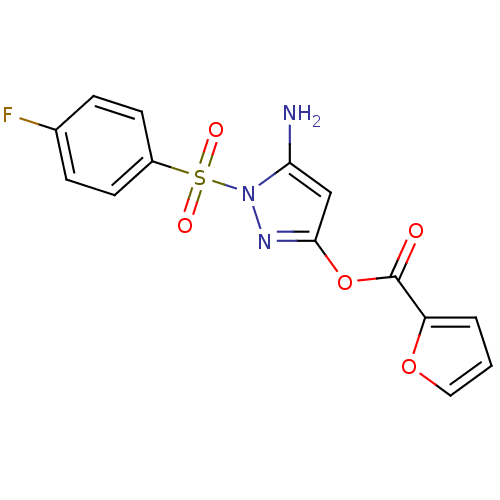 (2-furancarboxylic acid [5-amino-1-(4-fluorophenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Molecular Library Screening Center Curated by PubChem BioAssay | Assay Description The HTS assay to identify Inhibitors of West Nile Virus (WNV) NS2bNS3 Proteinase was proposed by Dr Alex Strongin of the Burnham Institute XO1-MH0776... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2222S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109048 (CHEMBL3601347) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109112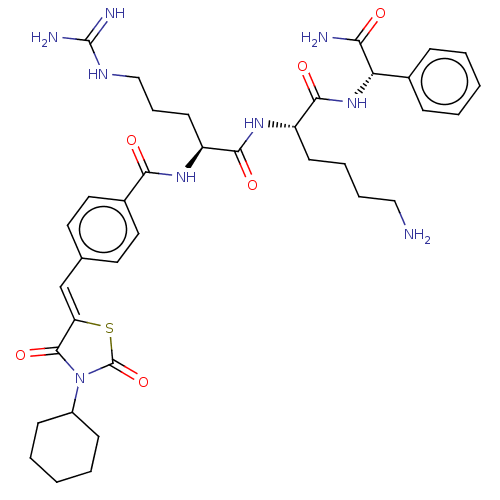 (CHEMBL3601350) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109265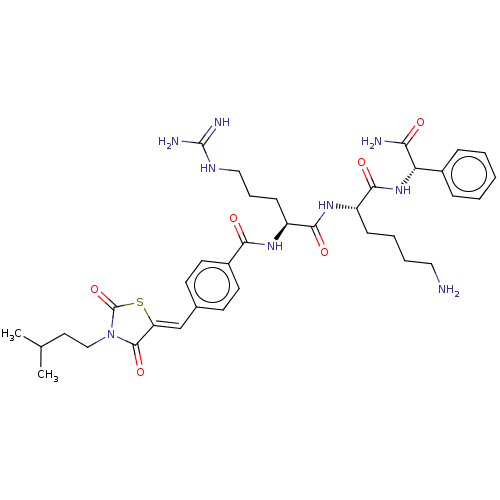 (CHEMBL3601102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50542820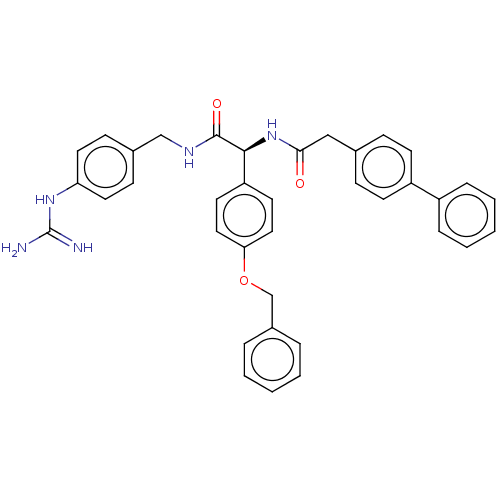 (CHEMBL4640021) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of West Nile virus NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as... | J Med Chem 63: 8179-8197 (2020) Article DOI: 10.1021/acs.jmedchem.0c00413 BindingDB Entry DOI: 10.7270/Q26H4N01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50567458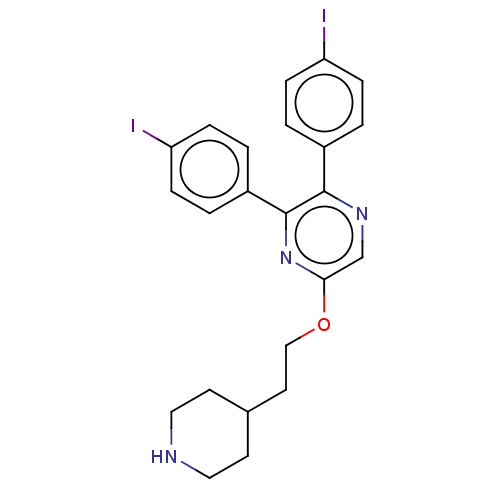 (CHEMBL4869904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition West Nile virus NS3B/NS3 protease using 7-amino-4-methylcoumarin as substrate by fluorescence assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02070 BindingDB Entry DOI: 10.7270/Q20G3PWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50302024 (5-amino-1-(4-methoxyphenylsulfonyl)-1H-pyrazol-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Conrad Prebys Center for Chemical Genomics Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease by fluorescence assay | Bioorg Med Chem Lett 19: 5773-7 (2009) Article DOI: 10.1016/j.bmcl.2009.07.150 BindingDB Entry DOI: 10.7270/Q2DZ08DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50542238 (CHEMBL4640778) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Inhibition of West Nile virus NS2B-NS3 protease | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126965 BindingDB Entry DOI: 10.7270/Q27M0CGT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50109267 (CHEMBL3601104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University Curated by ChEMBL | Assay Description Inhibition of WNV NS2B-NS3 protease expressed in Escherichia coli BL21 lambda (DE3) cells using Abz-Gly-Leu-Lys-Arg-Gly-Gly-3-(NO2)Tyr as substrate p... | Bioorg Med Chem 23: 5748-55 (2015) Article DOI: 10.1016/j.bmc.2015.07.012 BindingDB Entry DOI: 10.7270/Q25B048V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 280 total ) | Next | Last >> |