

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Mus musculus) | BDBM36334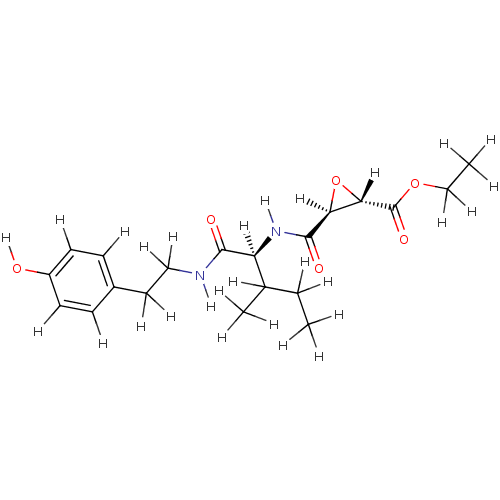 (CID644294 | JPM 565 | JPM-OEt | US9345789, JPM-Oet) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | 6.25 | 25 |
Stanford University | Assay Description IC50 measurements and enzyme kinetics assays were performed on a Spectramax M5 fluorescent plate reader (Molecular Devices). | ACS Chem Biol 5: 233-43 (2010) Article DOI: 10.1021/cb900232a BindingDB Entry DOI: 10.7270/Q2Z31X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Mus musculus) | BDBM50371947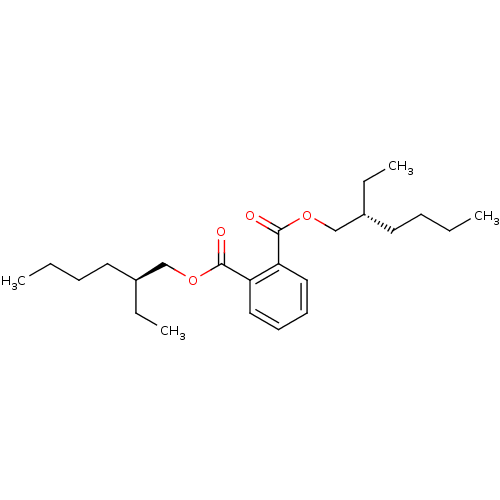 (BIS(2-ETHYLHEXYL)PHTHALATE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pukyong National University Curated by ChEMBL | Assay Description Inhibition of cathepsin B in mouse B16F10 cells | Bioorg Med Chem Lett 18: 2083-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.097 BindingDB Entry DOI: 10.7270/Q2TQ62CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Mus musculus) | BDBM50371946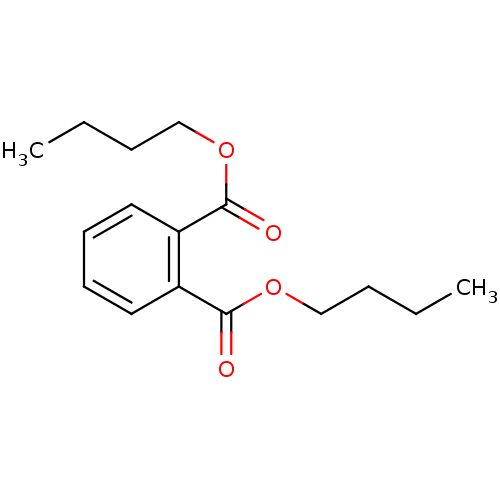 (CHEMBL272485) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pukyong National University Curated by ChEMBL | Assay Description Inhibition of cathepsin B in mouse B16F10 cells | Bioorg Med Chem Lett 18: 2083-8 (2008) Article DOI: 10.1016/j.bmcl.2008.01.097 BindingDB Entry DOI: 10.7270/Q2TQ62CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Mus musculus) | BDBM36332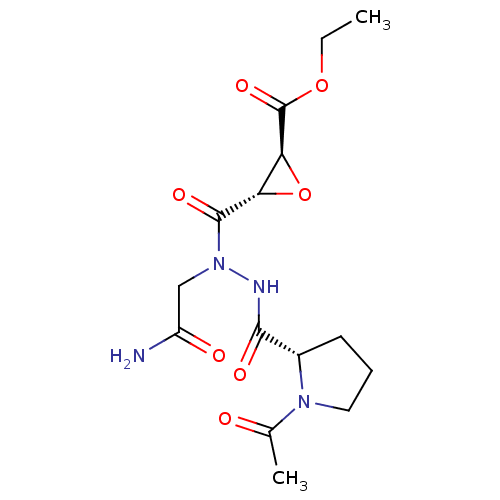 (LI-1 | Legumain Inhibitor -1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+5 | n/a | n/a | n/a | n/a | 6.25 | 25 |
Stanford University | Assay Description IC50 measurements and enzyme kinetics assays were performed on a Spectramax M5 fluorescent plate reader (Molecular Devices). | ACS Chem Biol 5: 233-43 (2010) Article DOI: 10.1021/cb900232a BindingDB Entry DOI: 10.7270/Q2Z31X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Mus musculus) | BDBM36333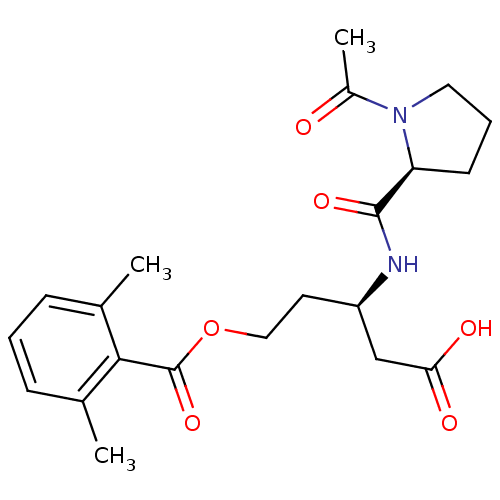 (LI-0 | Legumain Inhibitor -0) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | 6.25 | 25 |
Stanford University | Assay Description IC50 measurements and enzyme kinetics assays were performed on a Spectramax M5 fluorescent plate reader (Molecular Devices). | ACS Chem Biol 5: 233-43 (2010) Article DOI: 10.1021/cb900232a BindingDB Entry DOI: 10.7270/Q2Z31X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||