 Found 103 hits of ic50 for UniProtKB: P00403
Found 103 hits of ic50 for UniProtKB: P00403 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111601
BindingDB Entry DOI: 10.7270/Q20V8H5Q |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50140321
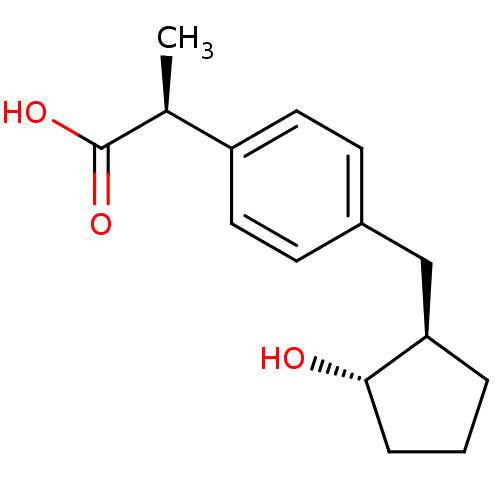
((S)-2-[4-((1R,2S)-2-Hydroxy-cyclopentylmethyl)-phe...)Show InChI InChI=1S/C15H20O3/c1-10(15(17)18)12-7-5-11(6-8-12)9-13-3-2-4-14(13)16/h5-8,10,13-14,16H,2-4,9H2,1H3,(H,17,18)/t10-,13+,14-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114258
BindingDB Entry DOI: 10.7270/Q2KS6WMX |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50229774
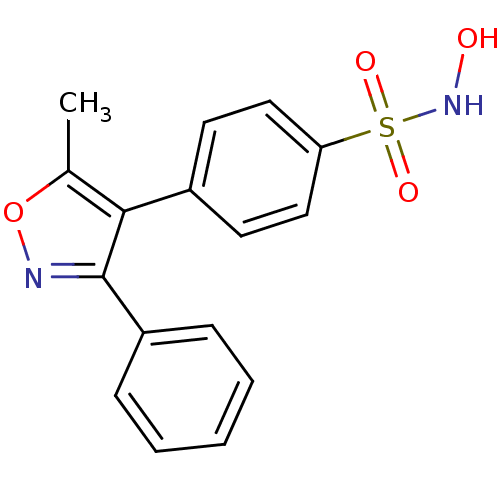
(CHEMBL254418 | N-Hydroxy-4-(5-methyl-3-phenylisoxa...)Show InChI InChI=1S/C16H14N2O4S/c1-11-15(16(17-22-11)13-5-3-2-4-6-13)12-7-9-14(10-8-12)23(20,21)18-19/h2-10,18-19H,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cyclooxygenase 2 |
Bioorg Med Chem 16: 5322-30 (2008)
Article DOI: 10.1016/j.bmc.2008.02.088
BindingDB Entry DOI: 10.7270/Q2FT8KS9 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human COX2 expressed in baculovirus infected Sf21 cells using arachidonic acid as substrate preincubated for 5 mins followe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111863
BindingDB Entry DOI: 10.7270/Q27S7SFQ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598758
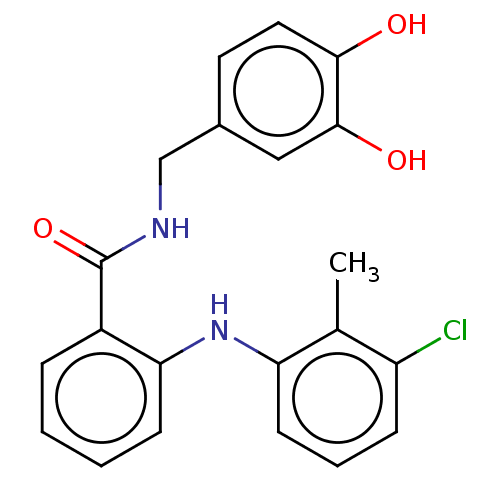
(CHEMBL5199117)Show SMILES Cc1c(Cl)cccc1Nc1ccccc1C(=O)NCc1ccc(O)c(O)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598765

(CHEMBL5207189)Show SMILES Cc1c(Cl)cccc1Nc1ccc(F)cc1C(=O)NCc1ccc(O)c(O)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human COX2 expressed in baculovirus infected Sf21 cells using arachidonic acid as substrate preincubated for 5 mins followe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111863
BindingDB Entry DOI: 10.7270/Q27S7SFQ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111601
BindingDB Entry DOI: 10.7270/Q20V8H5Q |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50140321

((S)-2-[4-((1R,2S)-2-Hydroxy-cyclopentylmethyl)-phe...)Show InChI InChI=1S/C15H20O3/c1-10(15(17)18)12-7-5-11(6-8-12)9-13-3-2-4-14(13)16/h5-8,10,13-14,16H,2-4,9H2,1H3,(H,17,18)/t10-,13+,14-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114258
BindingDB Entry DOI: 10.7270/Q2KS6WMX |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50515743
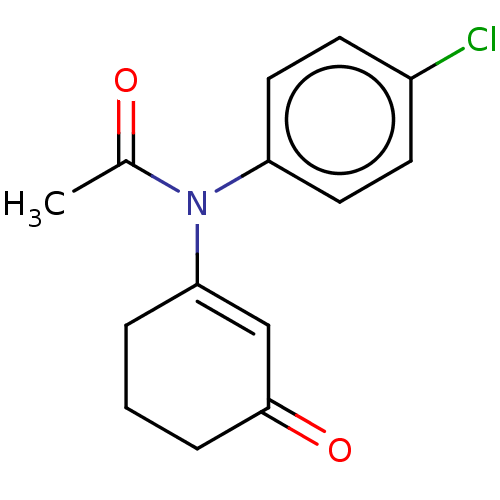
(CHEMBL4448990)Show InChI InChI=1S/C14H14ClNO2/c1-10(17)16(12-7-5-11(15)6-8-12)13-3-2-4-14(18)9-13/h5-9H,2-4H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 367 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111601
BindingDB Entry DOI: 10.7270/Q20V8H5Q |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50140321

((S)-2-[4-((1R,2S)-2-Hydroxy-cyclopentylmethyl)-phe...)Show InChI InChI=1S/C15H20O3/c1-10(15(17)18)12-7-5-11(6-8-12)9-13-3-2-4-14(13)16/h5-8,10,13-14,16H,2-4,9H2,1H3,(H,17,18)/t10-,13+,14-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114258
BindingDB Entry DOI: 10.7270/Q2KS6WMX |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598750
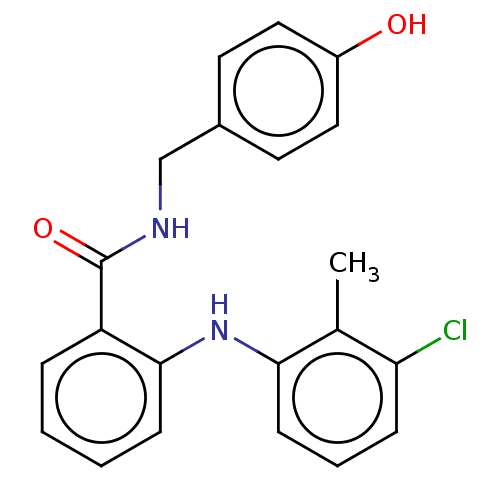
(CHEMBL5193981) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50515744

(CHEMBL4441570)Show InChI InChI=1S/C12H12ClNO/c13-9-4-6-10(7-5-9)14-11-2-1-3-12(15)8-11/h4-8,14H,1-3H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111601
BindingDB Entry DOI: 10.7270/Q20V8H5Q |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Thromboxane (TXA2) receptor antagonist activity using human platelet |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598754

(CHEMBL5173923) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598757
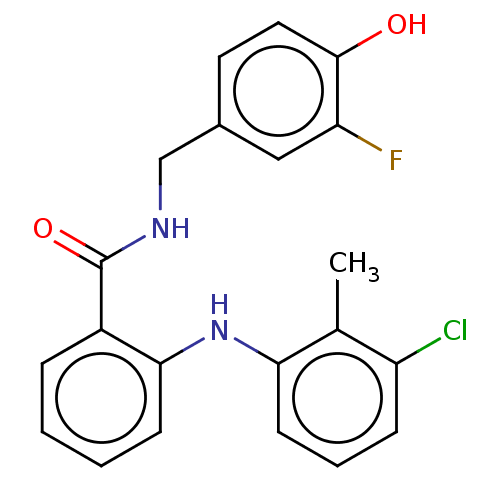
(CHEMBL5191029)Show SMILES Cc1c(Cl)cccc1Nc1ccccc1C(=O)NCc1ccc(O)c(F)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Antagonistic activity against TP-receptor by inhibition of U 46619-induced contraction of isolated guinea pig trachea |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50515745

(CHEMBL4474644)Show SMILES CC(C)(C)OC(=O)N(C1=CC(=O)CCC1)c1ccc(Cl)cc1 |t:8| Show InChI InChI=1S/C17H20ClNO3/c1-17(2,3)22-16(21)19(13-9-7-12(18)8-10-13)14-5-4-6-15(20)11-14/h7-11H,4-6H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 915 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111601
BindingDB Entry DOI: 10.7270/Q20V8H5Q |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598766
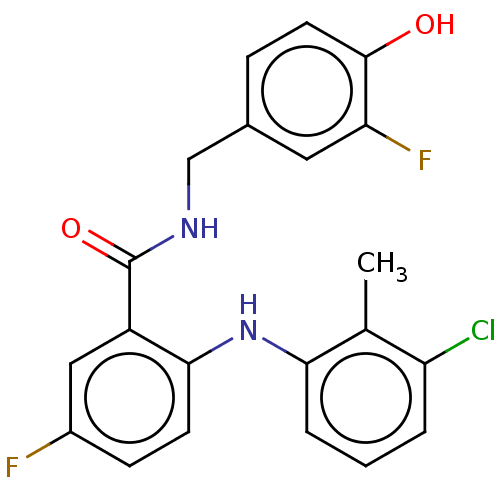
(CHEMBL5170447)Show SMILES Cc1c(Cl)cccc1Nc1ccc(F)cc1C(=O)NCc1ccc(O)c(F)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50558934
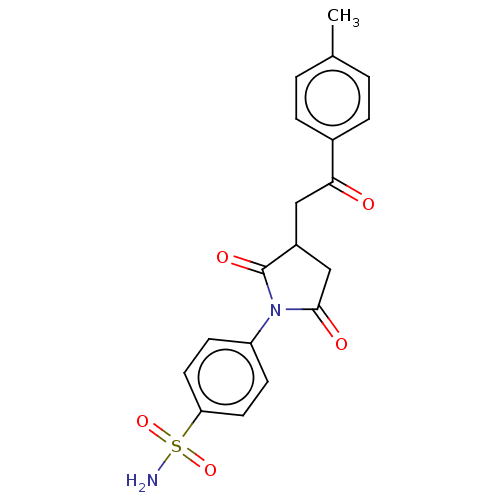
(CHEMBL4754218)Show SMILES Cc1ccc(cc1)C(=O)CC1CC(=O)N(C1=O)c1ccc(cc1)S(N)(=O)=O | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human COX2 expressed in baculovirus infected Sf21 cells using arachidonic acid as substrate preincubated for 5 mins followe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111863
BindingDB Entry DOI: 10.7270/Q27S7SFQ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cyclooxygenase 2 |
Bioorg Med Chem 16: 5322-30 (2008)
Article DOI: 10.1016/j.bmc.2008.02.088
BindingDB Entry DOI: 10.7270/Q2FT8KS9 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM35905
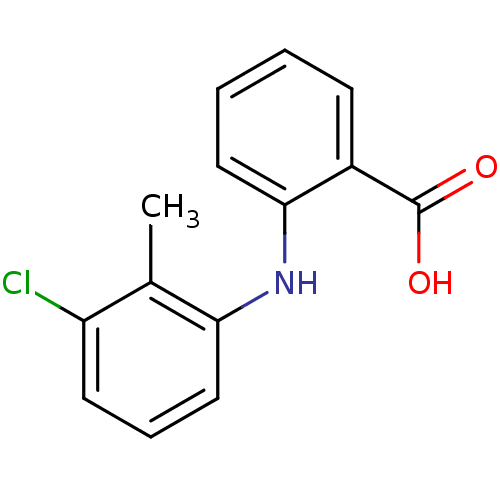
(Tolfenamic acid | cid_610479 | flufenamic acid ana...)Show InChI InChI=1S/C14H12ClNO2/c1-9-11(15)6-4-8-12(9)16-13-7-3-2-5-10(13)14(17)18/h2-8,16H,1H3,(H,17,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114560
BindingDB Entry DOI: 10.7270/Q2M90DNS |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50589148
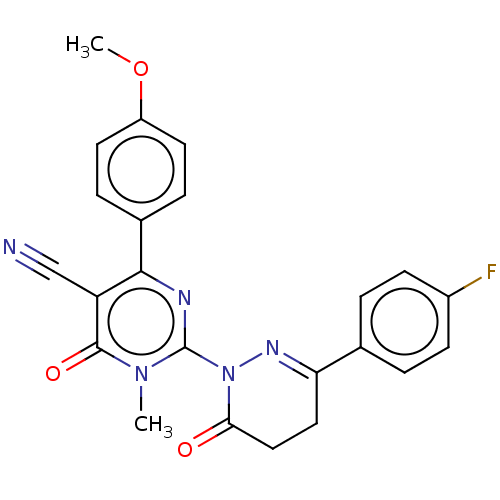
(CHEMBL5187847)Show SMILES COc1ccc(cc1)-c1nc(N2N=C(CCC2=O)c2ccc(F)cc2)n(C)c(=O)c1C#N |c:13| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112946
BindingDB Entry DOI: 10.7270/Q2WW7NNP |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM35905

(Tolfenamic acid | cid_610479 | flufenamic acid ana...)Show InChI InChI=1S/C14H12ClNO2/c1-9-11(15)6-4-8-12(9)16-13-7-3-2-5-10(13)14(17)18/h2-8,16H,1H3,(H,17,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50592551
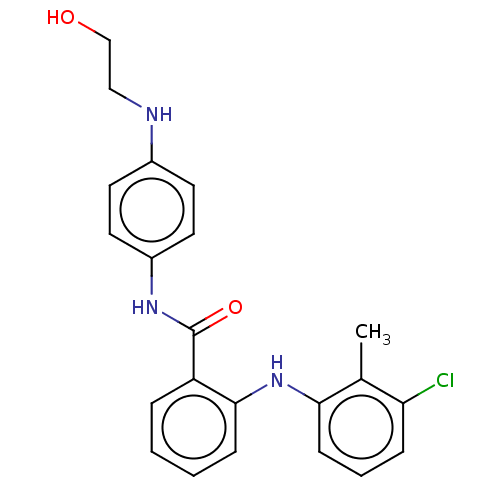
(CHEMBL5206236) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114560
BindingDB Entry DOI: 10.7270/Q2M90DNS |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598747

(CHEMBL5207774) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50558936

(CHEMBL4794507)Show SMILES COc1ccc(cc1)C(=O)CC1CC(=O)N(C1=O)c1ccc(cc1)S(N)(=O)=O | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human COX2 expressed in baculovirus infected Sf21 cells using arachidonic acid as substrate preincubated for 5 mins followe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111863
BindingDB Entry DOI: 10.7270/Q27S7SFQ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50592554
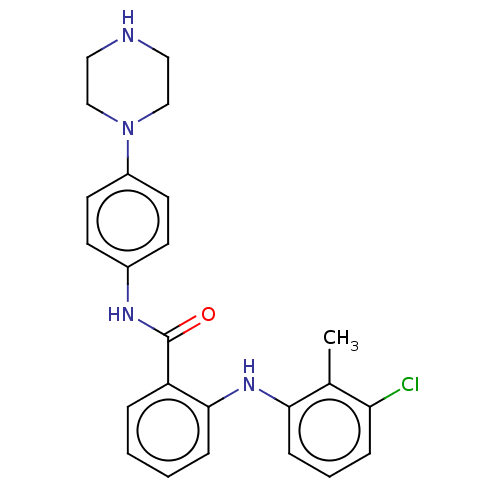
(CHEMBL5189018)Show SMILES Cc1c(Cl)cccc1Nc1ccccc1C(=O)Nc1ccc(cc1)N1CCNCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114560
BindingDB Entry DOI: 10.7270/Q2M90DNS |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Torino
Curated by ChEMBL
| Assay Description
Antagonistic activity against TP-receptor by inhibition of U 46619-induced contraction of isolated guinea pig trachea |
Eur J Med Chem 139: 936-946 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.046
BindingDB Entry DOI: 10.7270/Q2Q52S4X |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598752
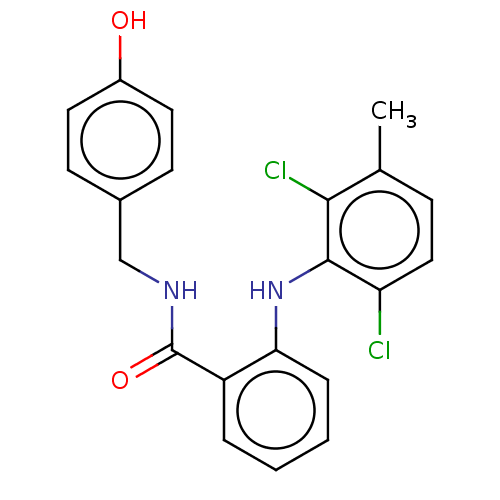
(CHEMBL5178874)Show SMILES Cc1ccc(Cl)c(Nc2ccccc2C(=O)NCc2ccc(O)cc2)c1Cl | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50592555
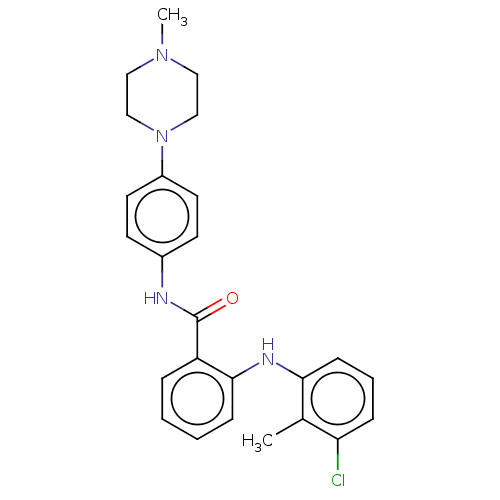
(CHEMBL5171524)Show SMILES CN1CCN(CC1)c1ccc(NC(=O)c2ccccc2Nc2cccc(Cl)c2C)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114560
BindingDB Entry DOI: 10.7270/Q2M90DNS |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50592557
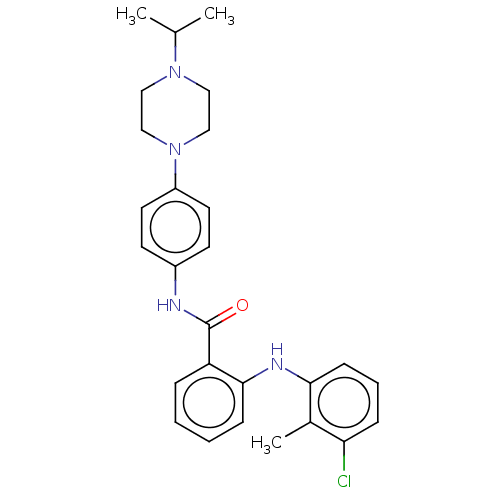
(CHEMBL5183520)Show SMILES CC(C)N1CCN(CC1)c1ccc(NC(=O)c2ccccc2Nc2cccc(Cl)c2C)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114560
BindingDB Entry DOI: 10.7270/Q2M90DNS |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50558937

(CHEMBL4779865)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(=O)CC(CC(=O)c2ccccc2)C1=O | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human COX2 expressed in baculovirus infected Sf21 cells using arachidonic acid as substrate preincubated for 5 mins followe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111863
BindingDB Entry DOI: 10.7270/Q27S7SFQ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50592556
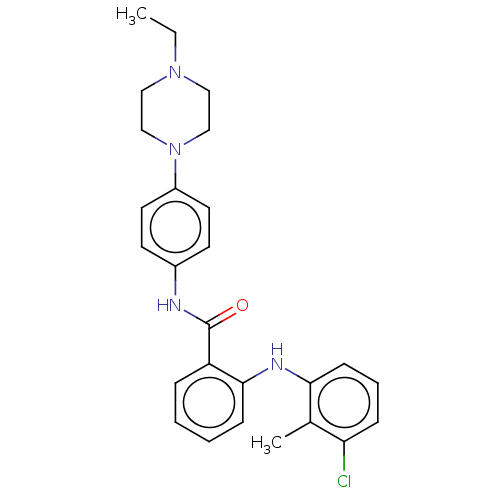
(CHEMBL5199286)Show SMILES CCN1CCN(CC1)c1ccc(NC(=O)c2ccccc2Nc2cccc(Cl)c2C)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114560
BindingDB Entry DOI: 10.7270/Q2M90DNS |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598751
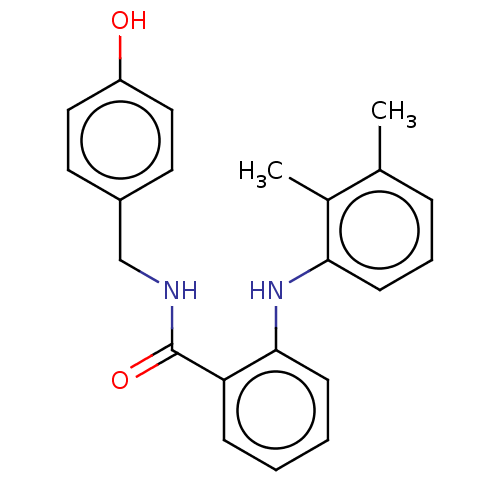
(CHEMBL5184694) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598756
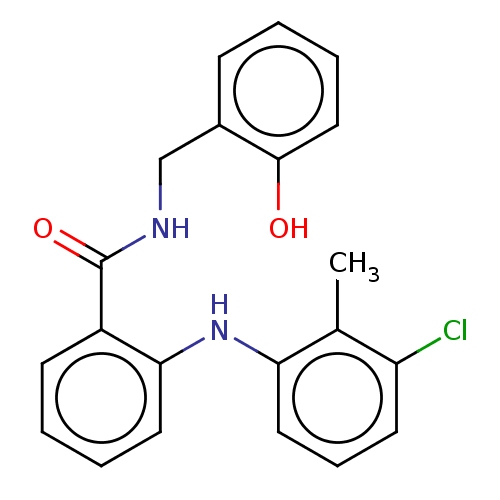
(CHEMBL5208621) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598753
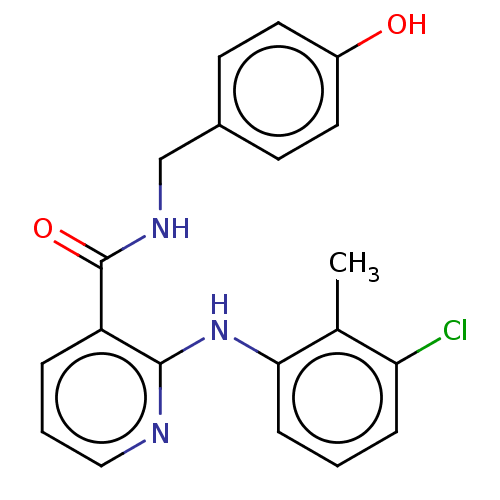
(CHEMBL5191175) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50558935
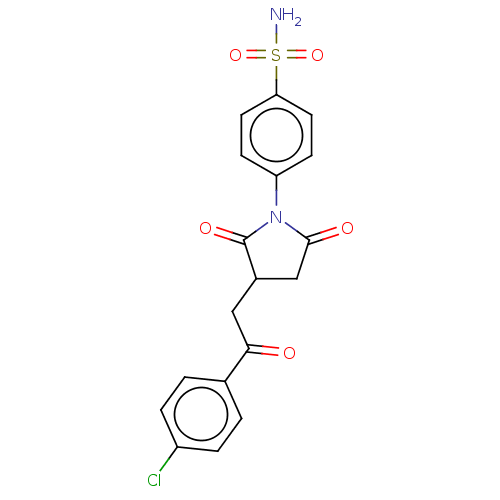
(CHEMBL4742170)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(=O)CC(CC(=O)c2ccc(Cl)cc2)C1=O | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human COX2 expressed in baculovirus infected Sf21 cells using arachidonic acid as substrate preincubated for 5 mins followe... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111863
BindingDB Entry DOI: 10.7270/Q27S7SFQ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50592563
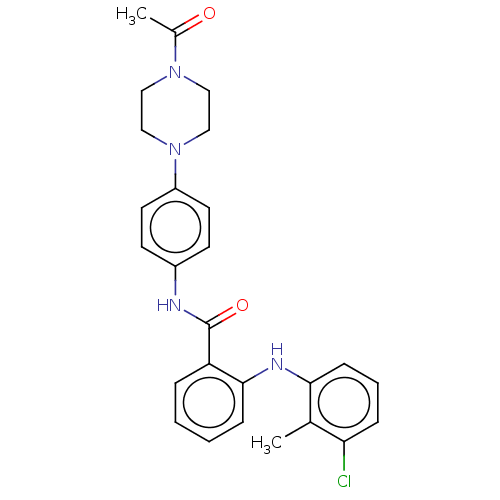
(CHEMBL5199367)Show SMILES CC(=O)N1CCN(CC1)c1ccc(NC(=O)c2ccccc2Nc2cccc(Cl)c2C)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114560
BindingDB Entry DOI: 10.7270/Q2M90DNS |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50598755
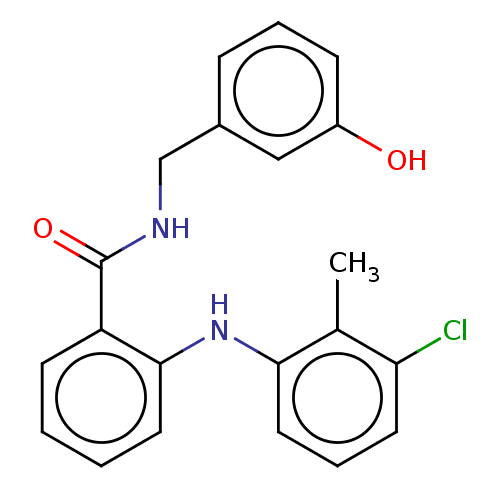
(CHEMBL5186581) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00635
BindingDB Entry DOI: 10.7270/Q27M0CZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50592558

(CHEMBL5188131)Show SMILES CCCN1CCN(CC1)c1ccc(NC(=O)c2ccccc2Nc2cccc(Cl)c2C)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114560
BindingDB Entry DOI: 10.7270/Q2M90DNS |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50592559

(CHEMBL5191274)Show SMILES CCCCN1CCN(CC1)c1ccc(NC(=O)c2ccccc2Nc2cccc(Cl)c2C)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114560
BindingDB Entry DOI: 10.7270/Q2M90DNS |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50595006
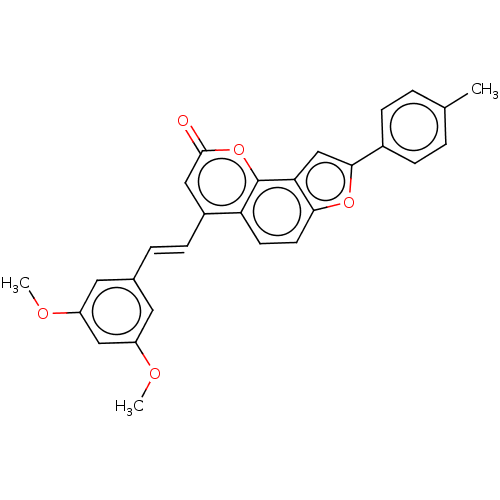
(CHEMBL5169638)Show SMILES COc1cc(OC)cc(\C=C\c2cc(=O)oc3c4cc(oc4ccc23)-c2ccc(C)cc2)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50595007
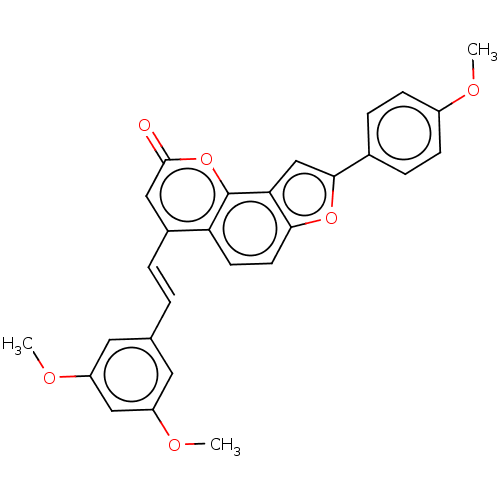
(CHEMBL5200035)Show SMILES COc1ccc(cc1)-c1cc2c(ccc3c(\C=C\c4cc(OC)cc(OC)c4)cc(=O)oc23)o1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50595000
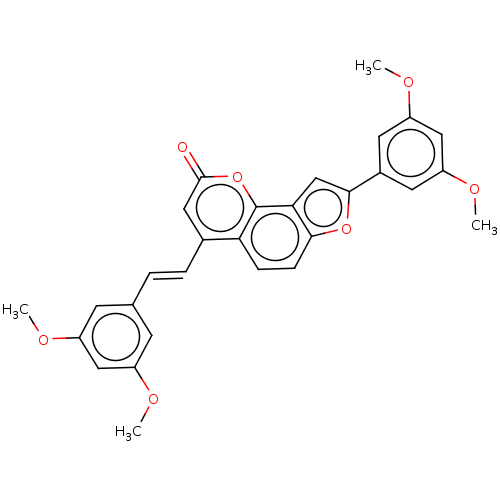
(CHEMBL5182136)Show SMILES COc1cc(OC)cc(\C=C\c2cc(=O)oc3c4cc(oc4ccc23)-c2cc(OC)cc(OC)c2)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50595001
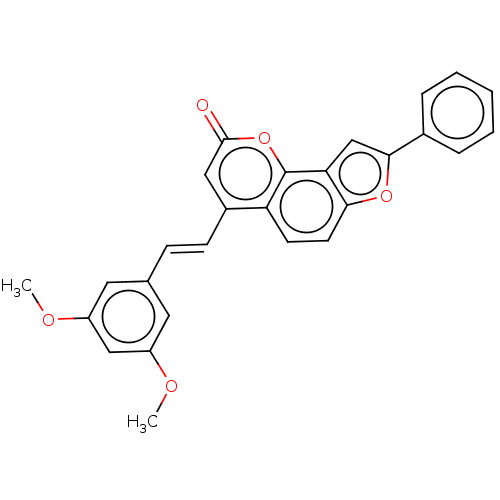
(CHEMBL5176910)Show SMILES COc1cc(OC)cc(\C=C\c2cc(=O)oc3c4cc(oc4ccc23)-c2ccccc2)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50595002
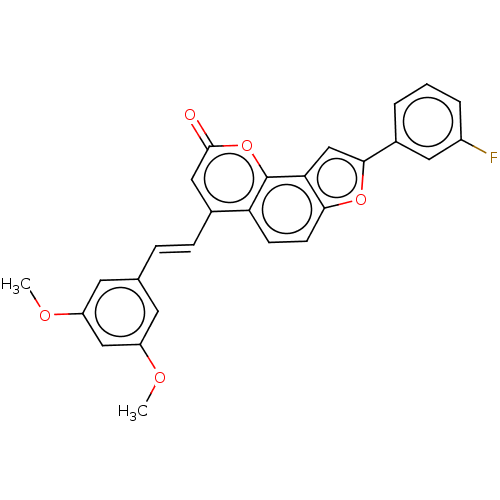
(CHEMBL5202541)Show SMILES COc1cc(OC)cc(\C=C\c2cc(=O)oc3c4cc(oc4ccc23)-c2cccc(F)c2)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50595003
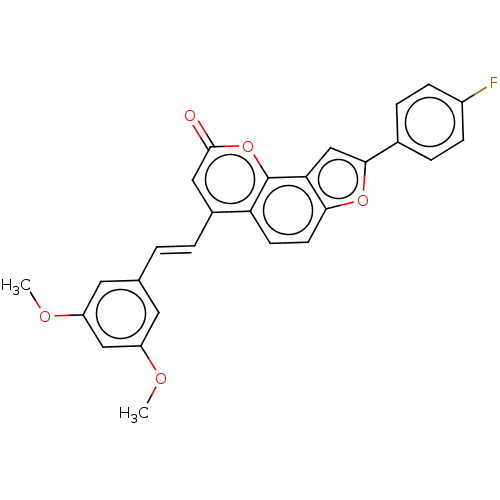
(CHEMBL5196941)Show SMILES COc1cc(OC)cc(\C=C\c2cc(=O)oc3c4cc(oc4ccc23)-c2ccc(F)cc2)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50595004
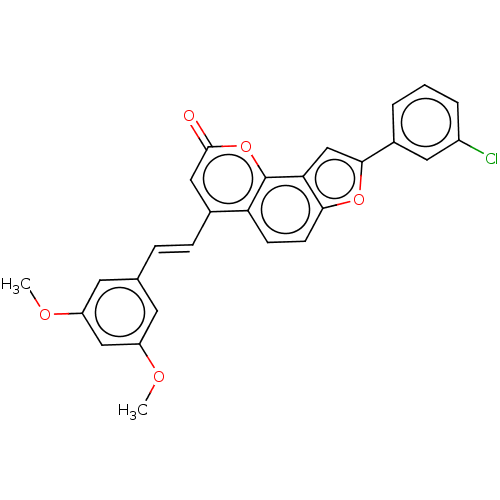
(CHEMBL5197349)Show SMILES COc1cc(OC)cc(\C=C\c2cc(=O)oc3c4cc(oc4ccc23)-c2cccc(Cl)c2)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit 2
(Homo sapiens (Human)) | BDBM50595005
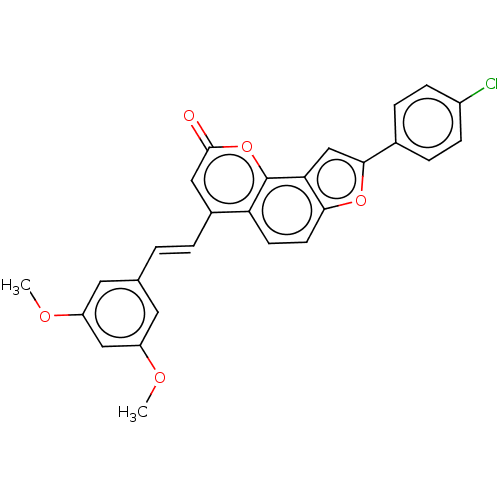
(CHEMBL5185240)Show SMILES COc1cc(OC)cc(\C=C\c2cc(=O)oc3c4cc(oc4ccc23)-c2ccc(Cl)cc2)c1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114242
BindingDB Entry DOI: 10.7270/Q2445RHZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data