 Found 32 hits of ic50 for UniProtKB: P29376
Found 32 hits of ic50 for UniProtKB: P29376 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM144315
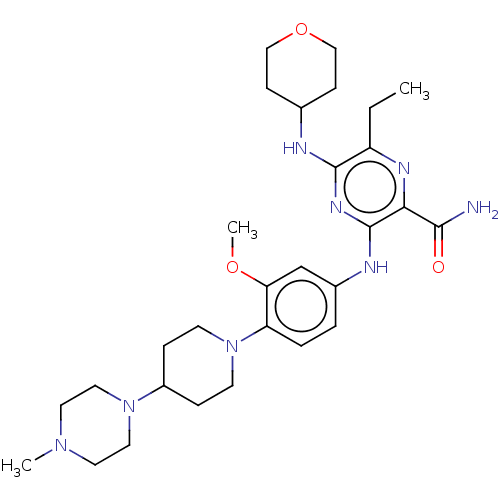
(Gilteritinib | US11512074, Example T-9 | US8969336...)Show SMILES CCc1nc(C(N)=O)c(Nc2ccc(N3CCC(CC3)N3CCN(C)CC3)c(OC)c2)nc1NC1CCOCC1 Show InChI InChI=1S/C29H44N8O3/c1-4-23-28(31-20-9-17-40-18-10-20)34-29(26(33-23)27(30)38)32-21-5-6-24(25(19-21)39-3)37-11-7-22(8-12-37)36-15-13-35(2)14-16-36/h5-6,19-20,22H,4,7-18H2,1-3H3,(H2,30,38)(H2,31,32,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01851
BindingDB Entry DOI: 10.7270/Q25Q515Z |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50512318
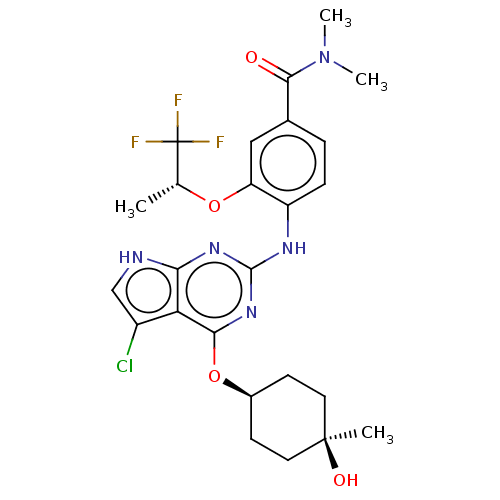
(CHEMBL4546504)Show SMILES C[C@@H](Oc1cc(ccc1Nc1nc(O[C@H]2CC[C@@](C)(O)CC2)c2c(Cl)c[nH]c2n1)C(=O)N(C)C)C(F)(F)F |r,wU:17.19,14.14,1.0,(59.2,-50.35,;60.54,-51.12,;61.87,-50.34,;61.86,-48.8,;60.53,-48.04,;60.52,-46.49,;61.86,-45.72,;63.19,-46.49,;63.18,-48.03,;64.52,-48.8,;65.85,-48.03,;65.85,-46.49,;67.18,-45.72,;67.18,-44.18,;65.84,-43.41,;64.51,-44.19,;63.18,-43.42,;63.17,-41.87,;62.4,-40.53,;61.63,-41.87,;64.51,-41.11,;65.84,-41.87,;68.52,-46.48,;69.98,-46,;70.45,-44.53,;70.89,-47.24,;70,-48.49,;68.53,-48.02,;67.18,-48.8,;59.19,-45.72,;59.19,-44.18,;57.86,-46.49,;56.52,-45.72,;57.86,-48.03,;60.54,-52.66,;59.21,-53.43,;61.88,-53.42,;60.53,-54.19,)| Show InChI InChI=1S/C25H29ClF3N5O4/c1-13(25(27,28)29)37-18-11-14(22(35)34(3)4)5-6-17(18)31-23-32-20-19(16(26)12-30-20)21(33-23)38-15-7-9-24(2,36)10-8-15/h5-6,11-13,15,36H,7-10H2,1-4H3,(H2,30,31,32,33)/t13-,15-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.567 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LTK cytoplasmic domain (450 to 864 residues) expressed in baculovirus expression system by Z'-LYTE assay |
J Med Chem 62: 4401-4410 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01869
BindingDB Entry DOI: 10.7270/Q2KS6VVN |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50448785
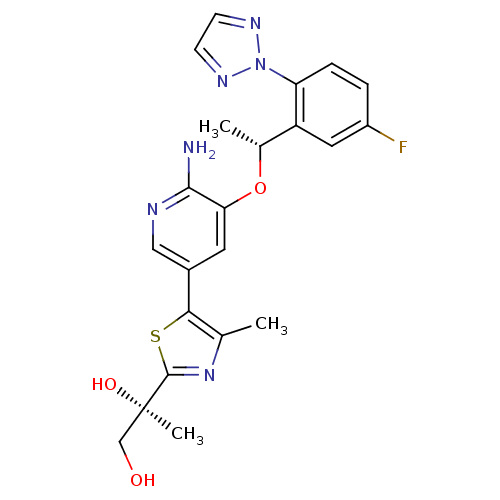
(CHEMBL3128069)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1sc(nc1C)[C@](C)(O)CO)c1cc(F)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H23FN6O3S/c1-12-19(33-21(28-12)22(3,31)11-30)14-8-18(20(24)25-10-14)32-13(2)16-9-15(23)4-5-17(16)29-26-6-7-27-29/h4-10,13,30-31H,11H2,1-3H3,(H2,24,25)/t13-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LTK (unknown origin) using Km levels of ATP |
J Med Chem 57: 1170-87 (2014)
Article DOI: 10.1021/jm401805h
BindingDB Entry DOI: 10.7270/Q29C6ZX5 |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50018830
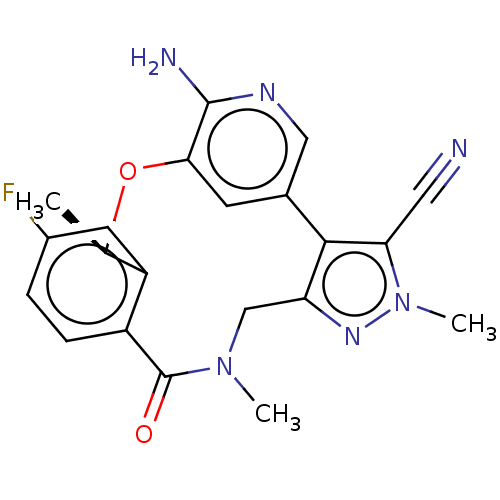
(CHEMBL3286830 | US10543199, Compound PF-06463922 |...)Show SMILES C[C@H]1Oc2cc(cnc2N)-c2c(CN(C)C(=O)c3ccc(F)cc13)nn(C)c2C#N |r| Show InChI InChI=1S/C21H19FN6O2/c1-11-15-7-13(22)4-5-14(15)21(29)27(2)10-16-19(17(8-23)28(3)26-16)12-6-18(30-11)20(24)25-9-12/h4-7,9,11H,10H2,1-3H3,(H2,24,25)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LTK (unknown origin) by TR-FRET-based Z'-LYTE assay |
J Med Chem 57: 4720-44 (2014)
Article DOI: 10.1021/jm500261q
BindingDB Entry DOI: 10.7270/Q2K35W68 |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University
Curated by ChEMBL
| Assay Description
Inhibition of LTK (unknown origin) incubated for 1 hr by spectrophotometric analysis |
Bioorg Med Chem 24: 3483-93 (2016)
Article DOI: 10.1016/j.bmc.2016.05.057
BindingDB Entry DOI: 10.7270/Q29G5PQT |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50520589
(CHEMBL4473365)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(Nc2ccccc2S(=O)(=O)C(C)C)n1 Show InChI InChI=1S/C28H36ClN7O4S/c1-8-26(37)31-21-15-22(24(40-7)16-23(21)36(6)14-13-35(4)5)33-28-30-17-19(29)27(34-28)32-20-11-9-10-12-25(20)41(38,39)18(2)3/h8-12,15-18H,1,13-14H2,2-7H3,(H,31,37)(H2,30,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged human LTK cytoplasmic domain (440 to 864 residues) expressed in baculovirus expression system by Z-LYTE assay |
Eur J Med Chem 136: 497-510 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.079
BindingDB Entry DOI: 10.7270/Q2WH2TCG |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50554817
(CHEMBL4764610)Show SMILES COc1cc2c(c(F)c1N1C[C@H](C)N(C)[C@H](C)C1)n(C(C)C)c1[nH]c3cc(ccc3c1c2=O)C#N |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human LTK |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01550
BindingDB Entry DOI: 10.7270/Q2KK9GG7 |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human TYK1 using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human TYK1 using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50187716

(CHEMBL3735648)Show SMILES CC1(C)c2[nH]c3cc(ccc3c2C(=O)c2ccc(cc12)C1CCN(CC2CCOCC2)CC1)C#N Show InChI InChI=1S/C30H33N3O2/c1-30(2)25-16-22(21-7-11-33(12-8-21)18-19-9-13-35-14-10-19)4-6-23(25)28(34)27-24-5-3-20(17-31)15-26(24)32-29(27)30/h3-6,15-16,19,21,32H,7-14,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LTK (unknown origin) using poly(Glu,Tyr)4:1 as substrate incubated for 60 mins by ELISA |
Eur J Med Chem 118: 244-9 (2016)
Article DOI: 10.1016/j.ejmech.2016.04.046
BindingDB Entry DOI: 10.7270/Q2Q81G02 |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50240779
(CHEMBL4067871)Show SMILES OC[C@H](Nc1cnc(-c2cc(Cl)ccc2O)c(c1)-c1ccc2cnccc2c1)c1ccccc1 |r| Show InChI InChI=1S/C28H22ClN3O2/c29-22-8-9-27(34)25(13-22)28-24(20-6-7-21-15-30-11-10-19(21)12-20)14-23(16-31-28)32-26(17-33)18-4-2-1-3-5-18/h1-16,26,32-34H,17H2/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LTK (unknown origin) using poly (Glu,Tyr) 4:1 as substrate after 60 mins by ELISA |
J Med Chem 60: 6018-6035 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00076
BindingDB Entry DOI: 10.7270/Q2K939NC |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50240782
(CHEMBL4075917)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(N[C@@H](CO)c2ccccc2)cnc1-c1ccc(F)cc1O |r| Show InChI InChI=1S/C27H23FN4O2/c1-16-22-11-18(7-10-24(22)32-31-16)23-13-20(30-25(15-33)17-5-3-2-4-6-17)14-29-27(23)21-9-8-19(28)12-26(21)34/h2-14,25,30,33-34H,15H2,1H3,(H,31,32)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LTK (unknown origin) using poly (Glu,Tyr) 4:1 as substrate after 60 mins by ELISA |
J Med Chem 60: 6018-6035 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00076
BindingDB Entry DOI: 10.7270/Q2K939NC |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50240780

(CHEMBL4062877)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(N[C@@H](CO)c2ccccc2)cnc1-c1cc(F)ccc1O |r| Show InChI InChI=1S/C27H23FN4O2/c1-16-21-11-18(7-9-24(21)32-31-16)22-13-20(30-25(15-33)17-5-3-2-4-6-17)14-29-27(22)23-12-19(28)8-10-26(23)34/h2-14,25,30,33-34H,15H2,1H3,(H,31,32)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LTK (unknown origin) using poly (Glu,Tyr) 4:1 as substrate after 60 mins by ELISA |
J Med Chem 60: 6018-6035 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00076
BindingDB Entry DOI: 10.7270/Q2K939NC |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50240781
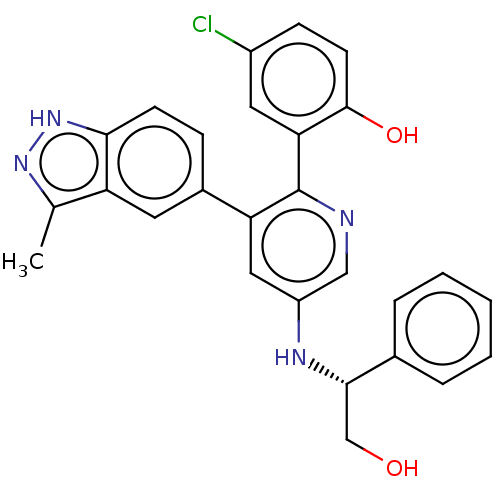
(CHEMBL4105329)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(N[C@@H](CO)c2ccccc2)cnc1-c1cc(Cl)ccc1O |r| Show InChI InChI=1S/C27H23ClN4O2/c1-16-21-11-18(7-9-24(21)32-31-16)22-13-20(30-25(15-33)17-5-3-2-4-6-17)14-29-27(22)23-12-19(28)8-10-26(23)34/h2-14,25,30,33-34H,15H2,1H3,(H,31,32)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LTK (unknown origin) using poly (Glu,Tyr) 4:1 as substrate after 60 mins by ELISA |
J Med Chem 60: 6018-6035 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00076
BindingDB Entry DOI: 10.7270/Q2K939NC |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50235830
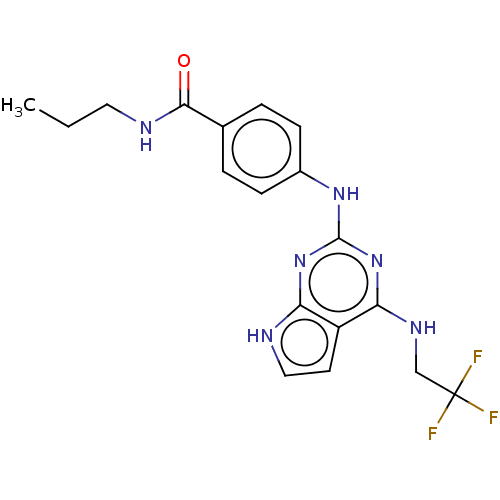
(CHEMBL2006765)Show SMILES CCCNC(=O)c1ccc(Nc2nc(NCC(F)(F)F)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C18H19F3N6O/c1-2-8-23-16(28)11-3-5-12(6-4-11)25-17-26-14-13(7-9-22-14)15(27-17)24-10-18(19,20)21/h3-7,9H,2,8,10H2,1H3,(H,23,28)(H3,22,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of LTK (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50322535
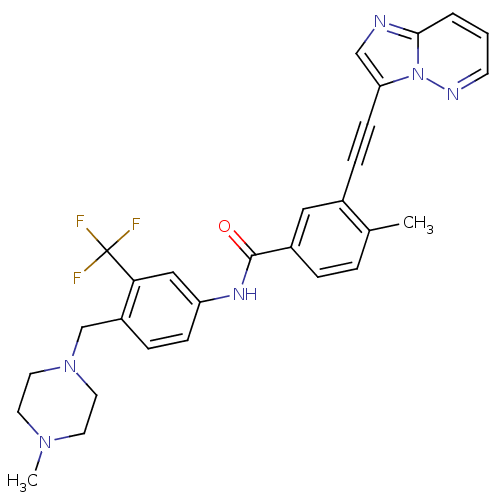
(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to LTK (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00081
BindingDB Entry DOI: 10.7270/Q2WM1J37 |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50235816

(CHEMBL4069365)Show SMILES CC1(C)OCC(=O)Nc2cc(Nc3nc(NCC(F)(F)F)c4occc4n3)ccc12 Show InChI InChI=1S/C19H18F3N5O3/c1-18(2)11-4-3-10(7-13(11)25-14(28)8-30-18)24-17-26-12-5-6-29-15(12)16(27-17)23-9-19(20,21)22/h3-7H,8-9H2,1-2H3,(H,25,28)(H2,23,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of LTK (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50235833
(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of LTK (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50235820
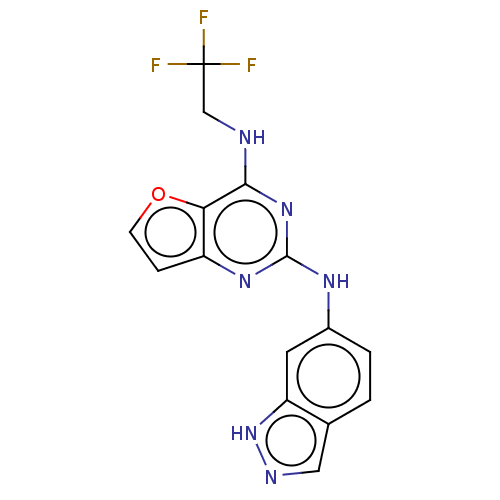
(CHEMBL4066664)Show InChI InChI=1S/C15H11F3N6O/c16-15(17,18)7-19-13-12-10(3-4-25-12)22-14(23-13)21-9-2-1-8-6-20-24-11(8)5-9/h1-6H,7H2,(H,20,24)(H2,19,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of LTK (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50235817
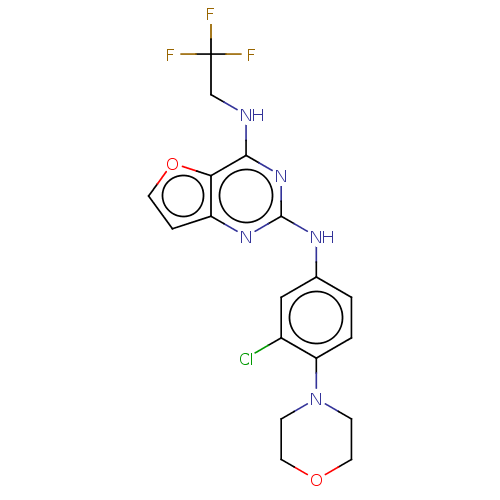
(CHEMBL4078893)Show SMILES FC(F)(F)CNc1nc(Nc2ccc(N3CCOCC3)c(Cl)c2)nc2ccoc12 Show InChI InChI=1S/C18H17ClF3N5O2/c19-12-9-11(1-2-14(12)27-4-7-28-8-5-27)24-17-25-13-3-6-29-15(13)16(26-17)23-10-18(20,21)22/h1-3,6,9H,4-5,7-8,10H2,(H2,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50509949
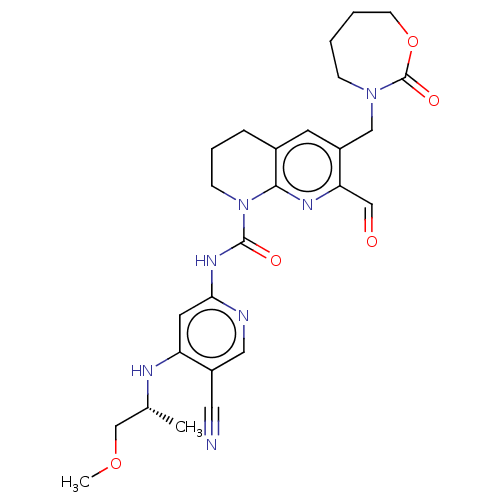
(CHEMBL4475216 | US11667631, Example 29)Show SMILES COC[C@@H](C)Nc1cc(NC(=O)N2CCCc3cc(CN4CCCCOC4=O)c(C=O)nc23)ncc1C#N |r| Show InChI InChI=1S/C26H31N7O5/c1-17(16-37-2)29-21-11-23(28-13-20(21)12-27)31-25(35)33-8-5-6-18-10-19(22(15-34)30-24(18)33)14-32-7-3-4-9-38-26(32)36/h10-11,13,15,17H,3-9,14,16H2,1-2H3,(H2,28,29,31,35)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University
Curated by ChEMBL
| Assay Description
Inhibition of LTK (unknown origin) |
Bioorg Med Chem 27: 1932-1941 (2019)
Article DOI: 10.1016/j.bmc.2019.04.018
BindingDB Entry DOI: 10.7270/Q2SB491W |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50135286

(CHEMBL3745885)Show SMILES Cn1c2nc(Nc3ccc4[nH]ccc4c3)ncc2cc(c1=O)S(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C22H15F2N5O3S/c1-29-20-13(9-19(21(29)30)33(31,32)18-5-2-14(23)10-16(18)24)11-26-22(28-20)27-15-3-4-17-12(8-15)6-7-25-17/h2-11,25H,1H3,(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of human TYK1 using [EAIYAAPFAKKK] as substrate |
Bioorg Med Chem 24: 521-44 (2016)
Article DOI: 10.1016/j.bmc.2015.11.045
BindingDB Entry DOI: 10.7270/Q24Q7WT8 |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50519662
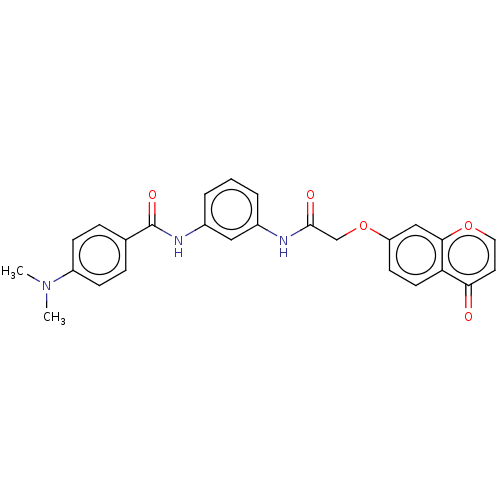
(CHEMBL4438748)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1cccc(NC(=O)COc2ccc3c(c2)occc3=O)c1 Show InChI InChI=1S/C26H23N3O5/c1-29(2)20-8-6-17(7-9-20)26(32)28-19-5-3-4-18(14-19)27-25(31)16-34-21-10-11-22-23(30)12-13-33-24(22)15-21/h3-15H,16H2,1-2H3,(H,27,31)(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human LTK (450 to end residues) using GEEPLYWSFPAKK as substrate after 40 mins in presence of [gamma-33ATP] by radiometric ... |
J Med Chem 62: 10691-10710 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01143
BindingDB Entry DOI: 10.7270/Q2MC93FG |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50306684
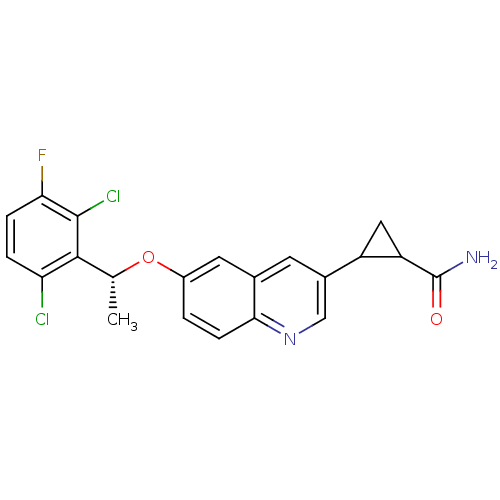
(2-(6-((R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy)qu...)Show SMILES C[C@@H](Oc1ccc2ncc(cc2c1)C1CC1C(N)=O)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H17Cl2FN2O2/c1-10(19-16(22)3-4-17(24)20(19)23)28-13-2-5-18-11(7-13)6-12(9-26-18)14-8-15(14)21(25)27/h2-7,9-10,14-15H,8H2,1H3,(H2,25,27)/t10-,14?,15?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LTK by TR-FRET assay |
Bioorg Med Chem Lett 20: 1405-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.109
BindingDB Entry DOI: 10.7270/Q2HQ401G |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50448449
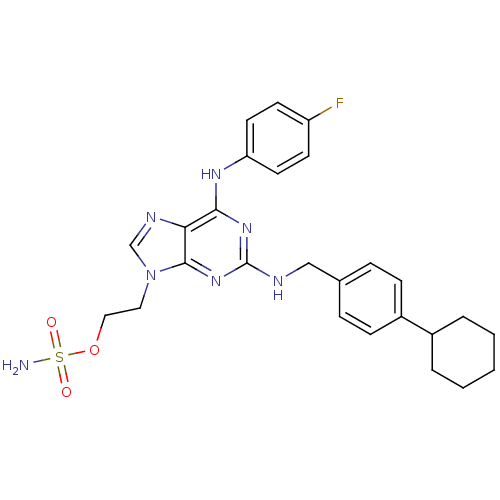
(CHEMBL3125667)Show SMILES NS(=O)(=O)OCCn1cnc2c(Nc3ccc(F)cc3)nc(NCc3ccc(cc3)C3CCCCC3)nc12 Show InChI InChI=1S/C26H30FN7O3S/c27-21-10-12-22(13-11-21)31-24-23-25(34(17-30-23)14-15-37-38(28,35)36)33-26(32-24)29-16-18-6-8-20(9-7-18)19-4-2-1-3-5-19/h6-13,17,19H,1-5,14-16H2,(H2,28,35,36)(H2,29,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of TYK1 (unknown origin) after 20 mins |
Bioorg Med Chem 21: 5618-28 (2013)
Article DOI: 10.1016/j.bmc.2013.04.080
BindingDB Entry DOI: 10.7270/Q2125V53 |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50306677
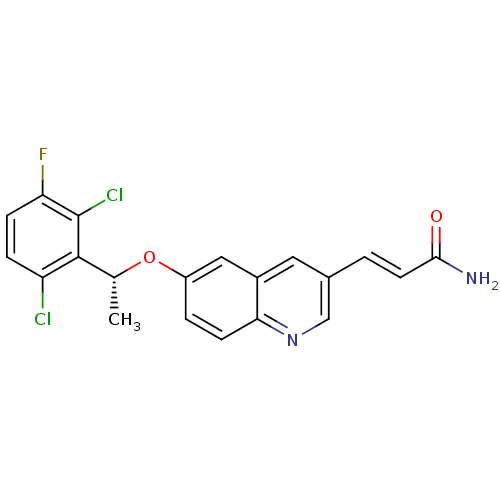
((R)-3-(6-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)qu...)Show SMILES C[C@@H](Oc1ccc2ncc(\C=C\C(N)=O)cc2c1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C20H15Cl2FN2O2/c1-11(19-15(21)4-5-16(23)20(19)22)27-14-3-6-17-13(9-14)8-12(10-25-17)2-7-18(24)26/h2-11H,1H3,(H2,24,26)/b7-2+/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LTK by TR-FRET assay |
Bioorg Med Chem Lett 20: 1405-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.109
BindingDB Entry DOI: 10.7270/Q2HQ401G |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50182493
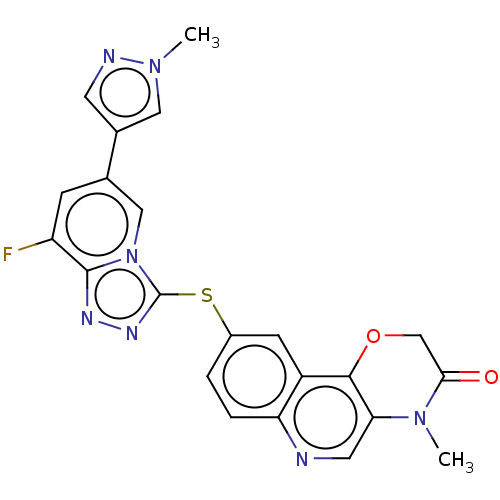
(CHEMBL3818247 | US9512121, 22)Show SMILES CN1C(=O)COc2c1cnc1ccc(Sc3nnc4c(F)cc(cn34)-c3cnn(C)c3)cc21 Show InChI InChI=1S/C22H16FN7O2S/c1-28-9-13(7-25-28)12-5-16(23)21-26-27-22(30(21)10-12)33-14-3-4-17-15(6-14)20-18(8-24-17)29(2)19(31)11-32-20/h3-10H,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southeast University
Curated by ChEMBL
| Assay Description
Inhibition of LTK (unknown origin) incubated for 1 hr by spectrophotometric analysis |
Bioorg Med Chem 24: 3483-93 (2016)
Article DOI: 10.1016/j.bmc.2016.05.057
BindingDB Entry DOI: 10.7270/Q29G5PQT |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM206104
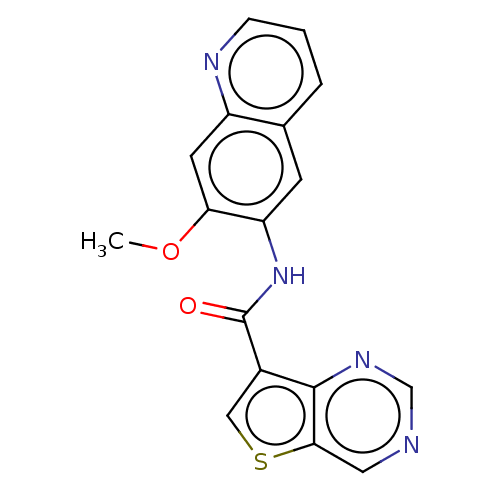
(US9255110, 17)Show InChI InChI=1S/C17H12N4O2S/c1-23-14-6-12-10(3-2-4-19-12)5-13(14)21-17(22)11-8-24-15-7-18-9-20-16(11)15/h2-9H,1H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged LTK cytoplasmic domain (498 to 796 residues) expressed in baculovirus expression system |
Bioorg Med Chem Lett 27: 1031-1036 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.064
BindingDB Entry DOI: 10.7270/Q22J6F3R |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50306683
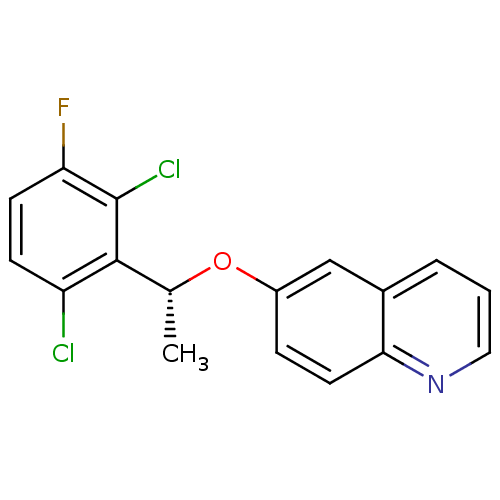
((R)-6-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)quino...)Show SMILES C[C@@H](Oc1ccc2ncccc2c1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C17H12Cl2FNO/c1-10(16-13(18)5-6-14(20)17(16)19)22-12-4-7-15-11(9-12)3-2-8-21-15/h2-10H,1H3/t10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant LTK by TR-FRET assay |
Bioorg Med Chem Lett 20: 1405-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.109
BindingDB Entry DOI: 10.7270/Q2HQ401G |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50364832
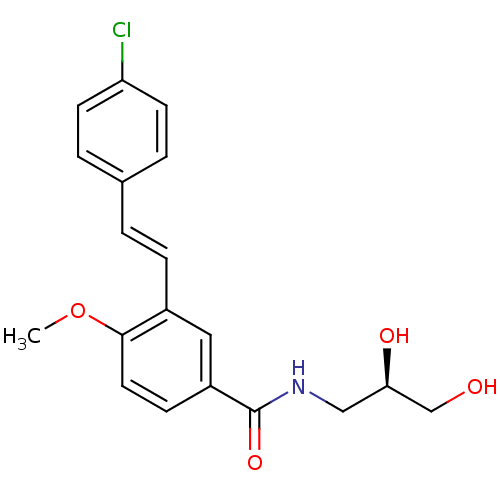
(CHEMBL1949788)Show SMILES COc1ccc(cc1\C=C\c1ccc(Cl)cc1)C(=O)NC[C@@H](O)CO |r| Show InChI InChI=1S/C19H20ClNO4/c1-25-18-9-6-15(19(24)21-11-17(23)12-22)10-14(18)5-2-13-3-7-16(20)8-4-13/h2-10,17,22-23H,11-12H2,1H3,(H,21,24)/b5-2+/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTK |
Bioorg Med Chem 20: 1442-60 (2012)
Article DOI: 10.1016/j.bmc.2011.12.058
BindingDB Entry DOI: 10.7270/Q2SB466W |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50364833
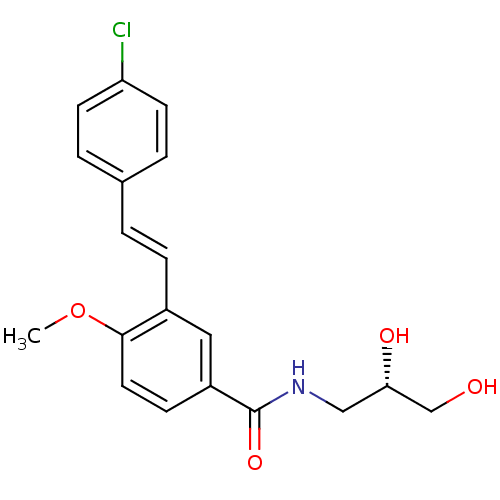
(CHEMBL1949789)Show SMILES COc1ccc(cc1\C=C\c1ccc(Cl)cc1)C(=O)NC[C@H](O)CO |r| Show InChI InChI=1S/C19H20ClNO4/c1-25-18-9-6-15(19(24)21-11-17(23)12-22)10-14(18)5-2-13-3-7-16(20)8-4-13/h2-10,17,22-23H,11-12H2,1H3,(H,21,24)/b5-2+/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LTK |
Bioorg Med Chem 20: 1442-60 (2012)
Article DOI: 10.1016/j.bmc.2011.12.058
BindingDB Entry DOI: 10.7270/Q2SB466W |
More data for this
Ligand-Target Pair | |
Leukocyte tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50537742
(CHEMBL4634634 | US11179389, Compound 1-14)Show SMILES C[C@@H]1C[C@H]1C(=O)N1CCN(C[C@H]1C)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H23N7O/c1-12-6-15(12)19(27)26-5-4-25(10-13(26)2)17-9-21-16(7-20)18(23-17)14-8-22-24(3)11-14/h8-9,11-13,15H,4-6,10H2,1-3H3/t12-,13-,15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged LTK (450 to 864 residues) cytoplasmic domain expressed in baculovirus expression system using tyrosine-6 p... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126715
BindingDB Entry DOI: 10.7270/Q2HM5CZG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data