

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human MST4 using MBP as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM50536679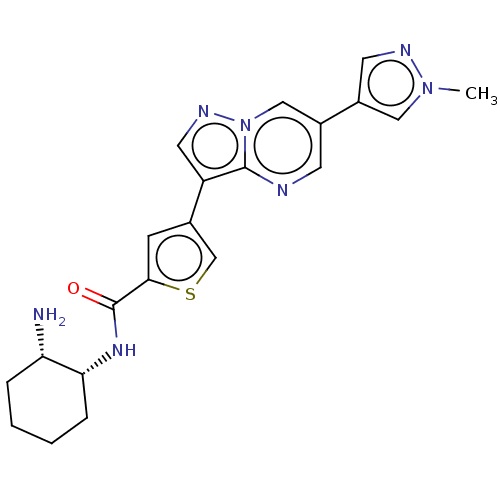 (CHEMBL4568087) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human GST-tagged MST4 expressed in baculovirus expression system by Z'-LYTE assay | Bioorg Med Chem Lett 26: 4362-6 (2016) Article DOI: 10.1016/j.bmcl.2016.02.003 BindingDB Entry DOI: 10.7270/Q2NP27X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM50442760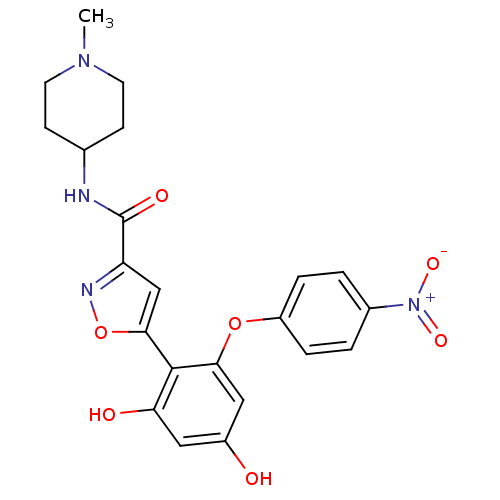 (CHEMBL2443044) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of MST4 (unknown origin) | Bioorg Med Chem 21: 7047-63 (2013) Article DOI: 10.1016/j.bmc.2013.09.018 BindingDB Entry DOI: 10.7270/Q2V989HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM50442758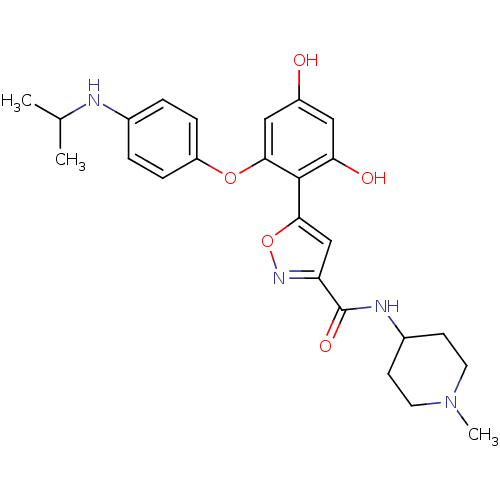 (CHEMBL2443139) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of MST4 (unknown origin) | Bioorg Med Chem 21: 7047-63 (2013) Article DOI: 10.1016/j.bmc.2013.09.018 BindingDB Entry DOI: 10.7270/Q2V989HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM50442757 (CHEMBL2443026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of MST4 (unknown origin) | Bioorg Med Chem 21: 7047-63 (2013) Article DOI: 10.1016/j.bmc.2013.09.018 BindingDB Entry DOI: 10.7270/Q2V989HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM50442759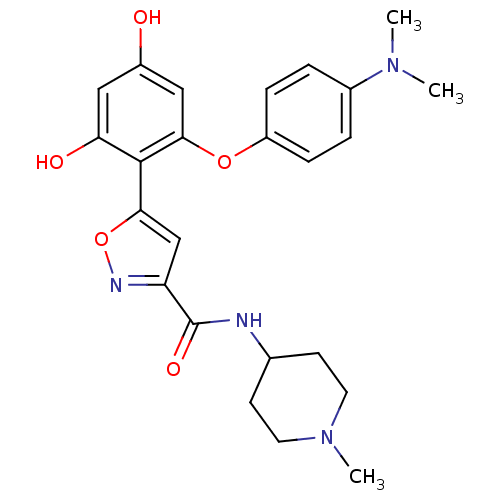 (CHEMBL2443138) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.r.l. Curated by ChEMBL | Assay Description Inhibition of MST4 (unknown origin) | Bioorg Med Chem 21: 7047-63 (2013) Article DOI: 10.1016/j.bmc.2013.09.018 BindingDB Entry DOI: 10.7270/Q2V989HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM50148921 (CHEMBL3770443) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant MST4 | ACS Med Chem Lett 6: 1241-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00398 BindingDB Entry DOI: 10.7270/Q2MP554V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 21.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MST4 using MBP as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM50536681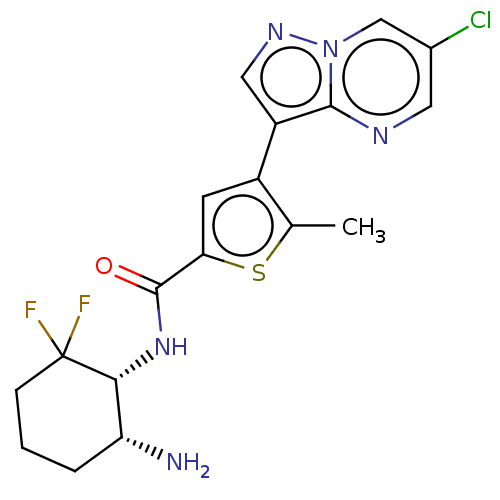 (CHEMBL4552628) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human GST-tagged MST4 expressed in baculovirus expression system by Z'-LYTE assay | Bioorg Med Chem Lett 26: 4362-6 (2016) Article DOI: 10.1016/j.bmcl.2016.02.003 BindingDB Entry DOI: 10.7270/Q2NP27X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM50059277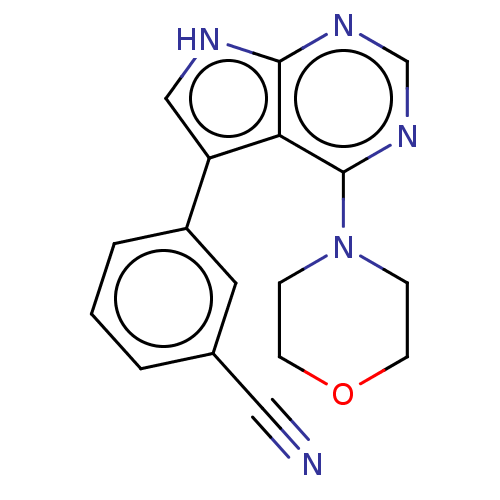 (CHEMBL3393348 | US9156845, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant MST4 using Ser/Thr peptide 7 substrate after 60 mins by Z-Lyte assay | J Med Chem 58: 419-32 (2015) Article DOI: 10.1021/jm5014055 BindingDB Entry DOI: 10.7270/Q2XS5X2W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM50536678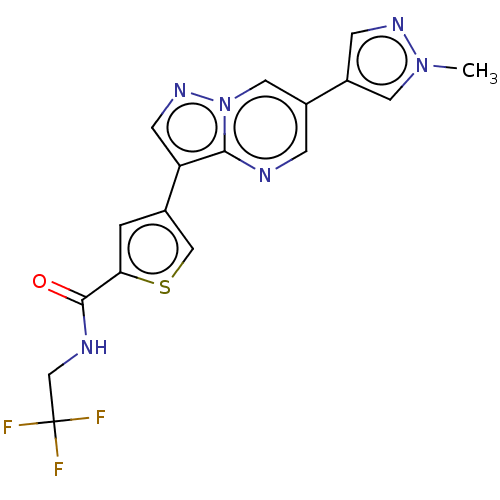 (CHEMBL4550702) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co. Curated by ChEMBL | Assay Description Inhibition of full-length recombinant human GST-tagged MST4 expressed in baculovirus expression system by Z'-LYTE assay | Bioorg Med Chem Lett 26: 4362-6 (2016) Article DOI: 10.1016/j.bmcl.2016.02.003 BindingDB Entry DOI: 10.7270/Q2NP27X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM50327912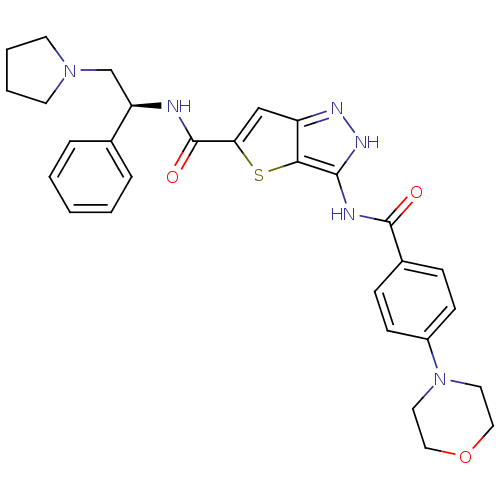 (3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology Curated by ChEMBL | Assay Description Inhibition of MST4 | Bioorg Med Chem 18: 7113-20 (2010) Article DOI: 10.1016/j.bmc.2010.07.048 BindingDB Entry DOI: 10.7270/Q2Q240GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451711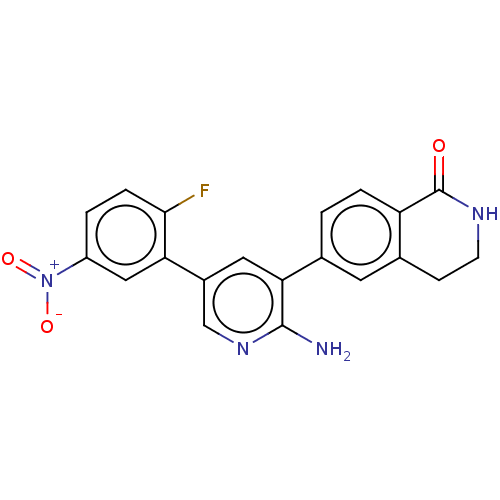 (6-(2-amino-5-(2-fluoro-5- nitrophenyl)pyridin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >900 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451763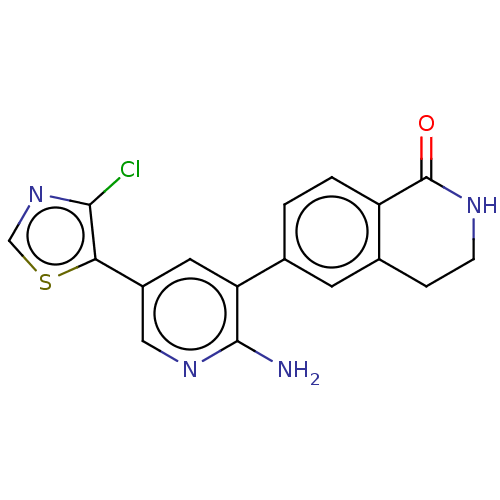 (6-(2-amino-5-(4- chlorothiazol-5-yl)pyridin-3- yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451712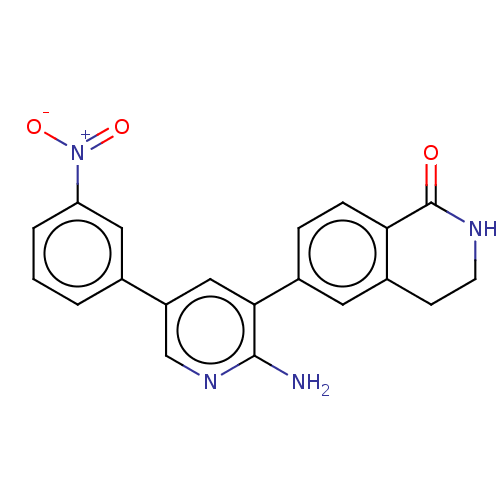 (6-(2-amino-5-(3- nitrophenyl)pyridin-3-yl)-3,4- di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451713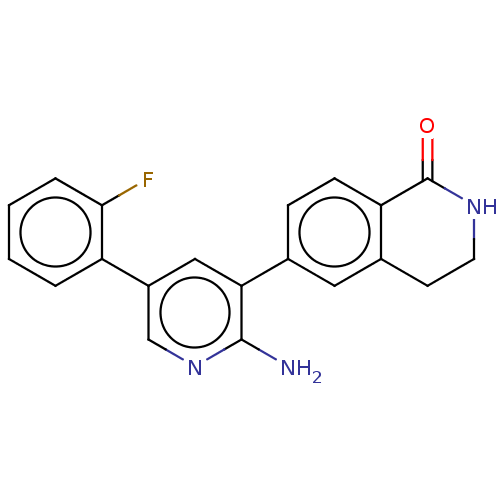 (6-(2-amino-5-(2- fluorophenyl)pyridin-3-yl)- 3,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451714 (6-(2-amino-5-(2-chloro-5- nitrophenyl)pyridin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451715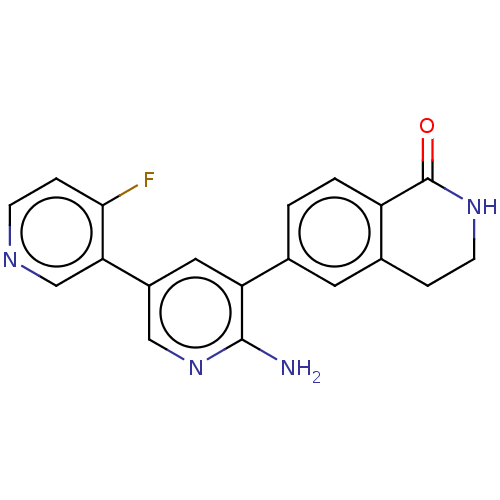 (6-(6-amino-4'-fluoro-[3,3'- bipyridin]-5-yl)-3,4- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451716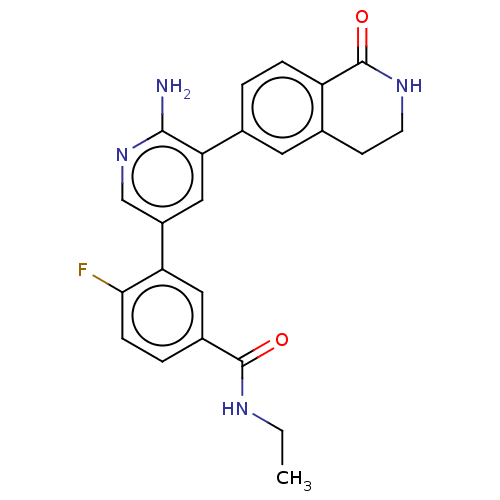 (3-(6-amino-5-(1-oxo-1,2,3,4- tetrahydroisoquinolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451717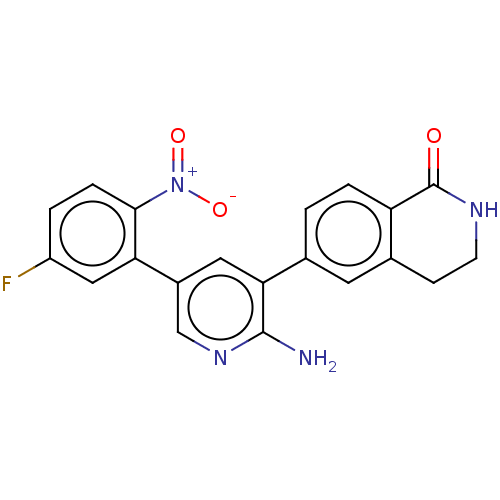 (6-(2-amino-5-(5-fluoro-2- nitrophenyl)pyridin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451718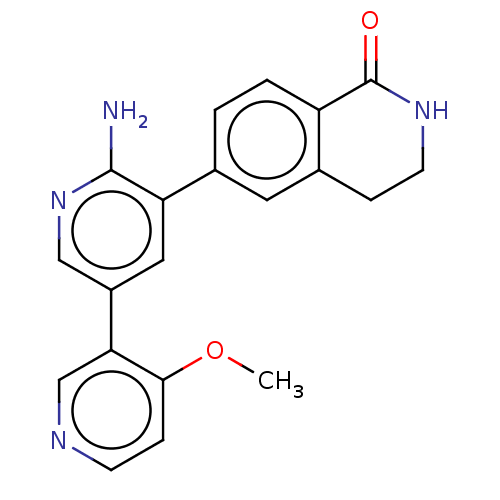 (6-(6-amino-4'-methoxy-[3,3'- bipyridin]-5-yl)-3,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451719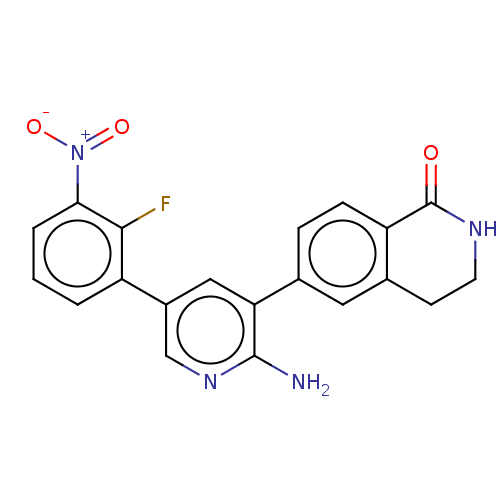 (6-(2-amino-5-(2-fluoro-3- nitrophenyl)pyridin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451721 (3-(6-amino-5-(1-oxo-1,2,3,4- tetrahydroisoquinolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451722 (6-(2-amino-5- (benzo[d]thiazol-7-yl)pyridin- 3-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451723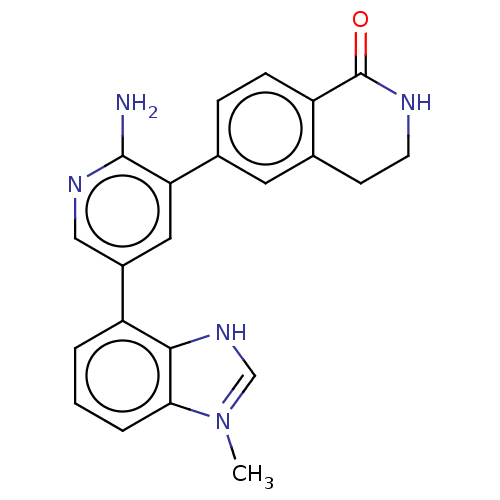 (6-(2-amino-5-(1-methyl-1H- benzo[d]imidazol-4- yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451724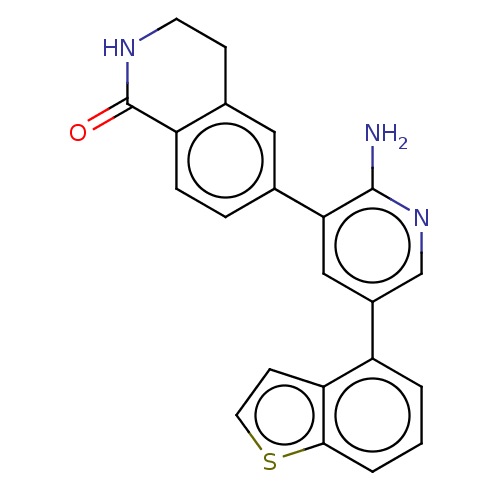 (6-(2-amino-5- (benzo[b]thiophen-4- yl)pyridin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451725 (6-(2-amino-5-(3-fluoro-5- nitrophenyl)pyridin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451726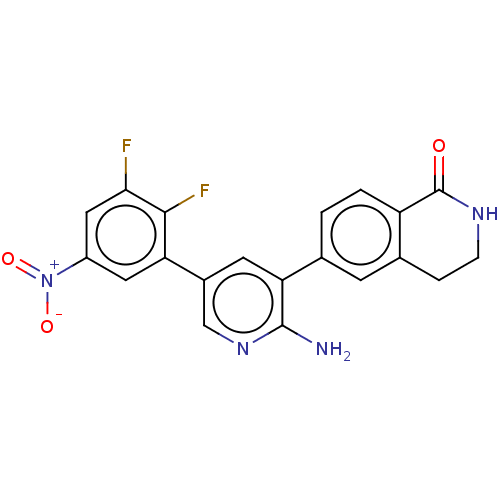 (6-(2-amino-5-(2,3-difluoro-5- nitrophenyl)pyridin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451727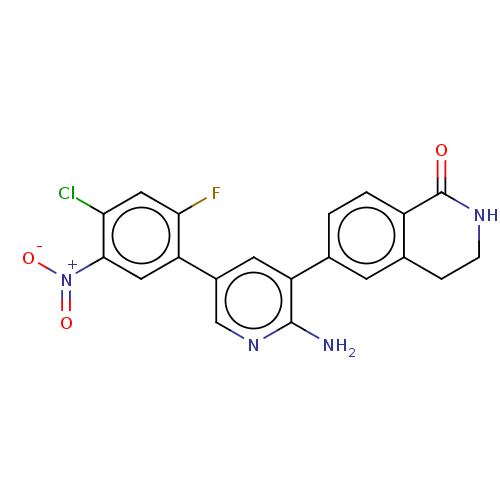 (6-(2-amino-5-(4-chloro-2- fluoro-5-nitrophenyl)pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451728 (6-(6-amino-5-(1-oxo-1,2,3,4- tetrahydroisoquinolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451729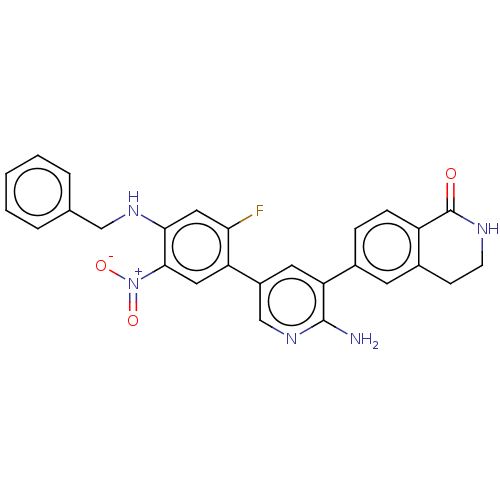 (6-(2-amino-5-(4- (benzylamino)-2-fluoro-5- nitroph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451731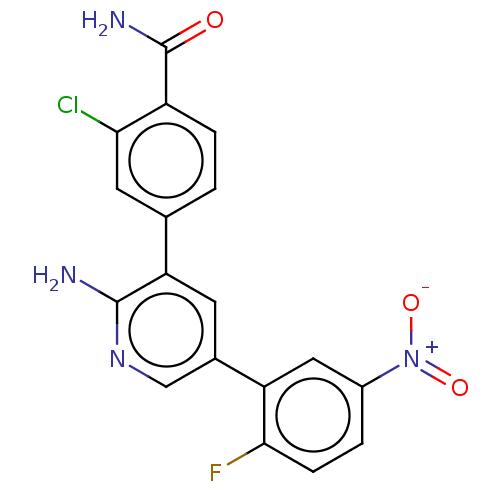 (4-(2-amino-5-(2-fluoro-5- nitrophenyl)pyridin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451732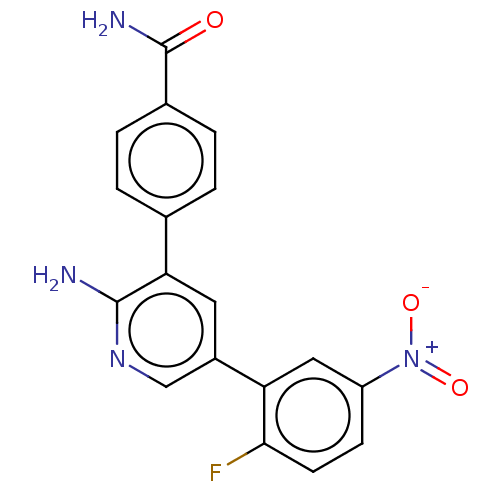 (4-(2-amino-5-(2-fluoro-5- nitrophenyl)pyridin-3- y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451733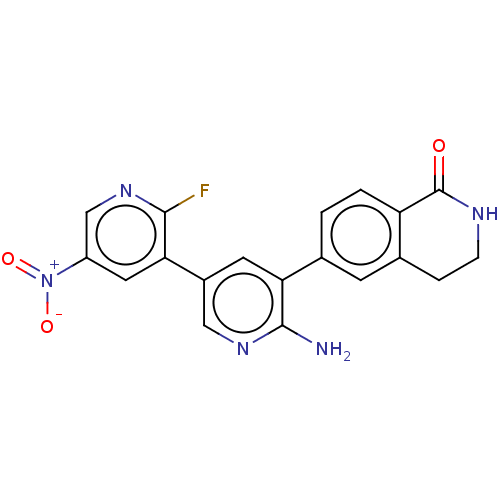 (6-(6-amino-2'-fluoro-5'-nitro- [3,3'-bipyridin]-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451735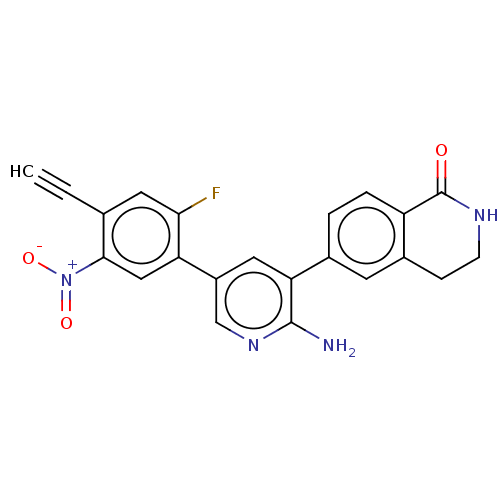 (6-(2-amino-5-(4-ethynyl-2- fluoro-5-nitrophenyl)py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451736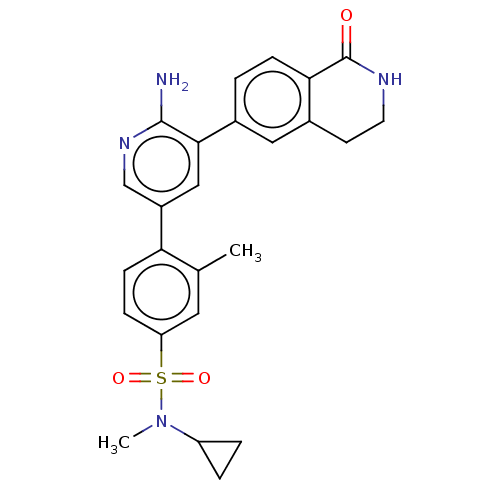 (4-(6-amino-5-(1-oxo-1,2,3,4- tetrahydroisoquinolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451737 (4-(6-amino-5-(1-oxo-1,2,3,4- tetrahydroisoquinolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451738 (4-(6-amino-5-(1-oxo-1,2,3,4- tetrahydroisoquinolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451739 (4-(6-amino-5-(1-oxo-1,2,3,4- tetrahydroisoquinolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451740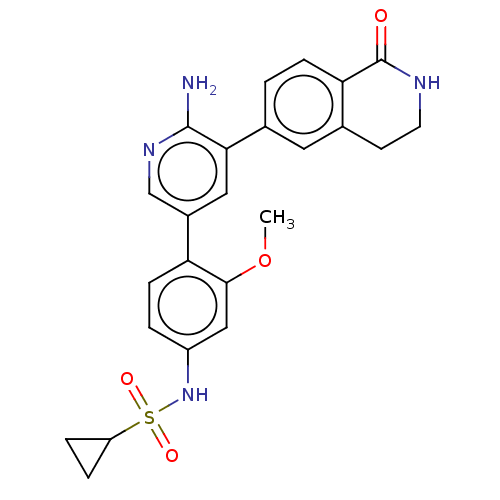 (N-(4-(6-amino-5-(1-oxo- 1,2,3,4-tetrahydroisoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451741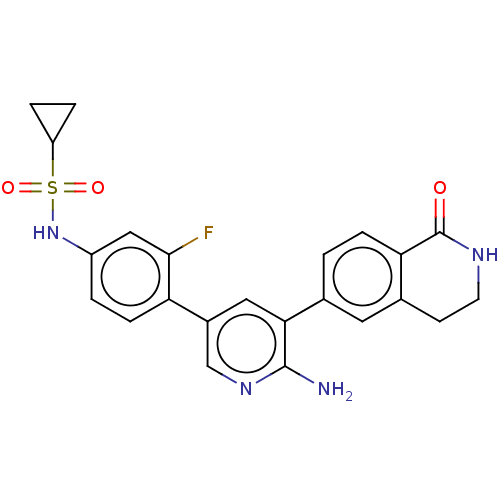 (N-(4-(6-amino-5-(1-oxo- 1,2,3,4-tetrahydroisoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451742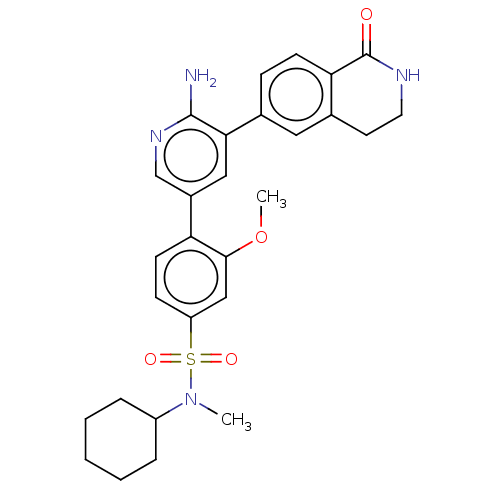 (4-(6-amino-5-(1-oxo-1,2,3,4- tetrahydroisoquinolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451743 (4-(6-amino-5-(1-oxo-1,2,3,4- tetrahydroisoquinolin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451744 (6-(2-amino-5-(2-fluoro-4- nitrophenyl)pyridin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451745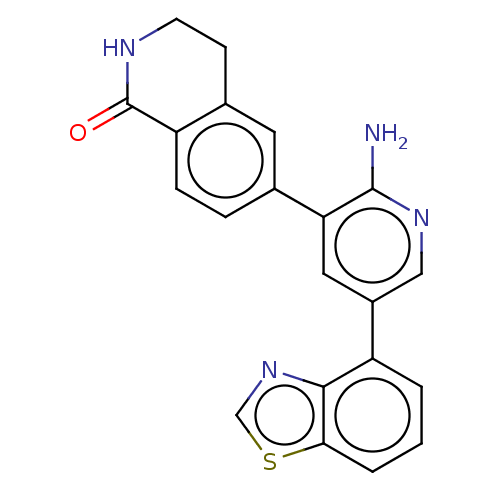 (6-(2-amino-5- (benzo[d]thiazol-4-yl)pyridin- 3-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451746 (6-(2-amino-5-(thiazolo[4,5- c]pyridin-7-yl)pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451747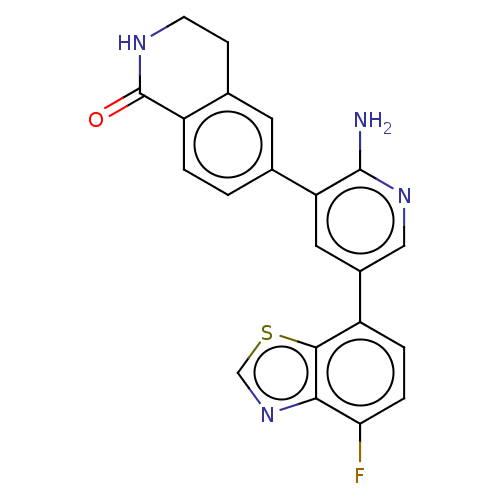 (6-(2-amino-5-(4- fluorobenzo[d]thiazol-7- yl)pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451748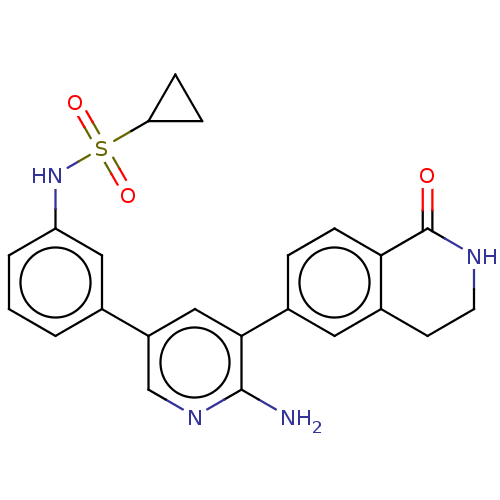 (N-(3-(6-amino-5-(1-oxo- 1,2,3,4-tetrahydroisoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451749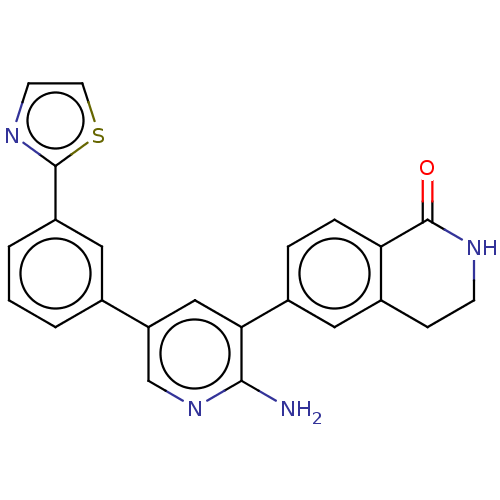 (6-(2-amino-5-(3-(thiazol-2- yl)phenyl)pyridin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 26 (Homo sapiens (Human)) | BDBM451750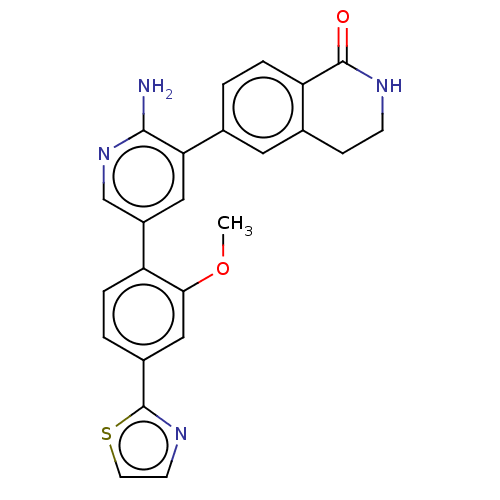 (6-(2-amino-5-(2-methoxy-4- (thiazol-2-yl)phenyl)py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DANA-FARBER CANCER INSTITUTE, INC.; FONDAZIONE CENTRO SAN RAFFAELE US Patent | Assay Description Biochemical IC50s were measured at Invitrogen using Z-lyte technology. Briefly, for STK4, the 2×STK4 (MST1)/Ser/Thr 07 mixture was prepared in 50 mM ... | US Patent US10710978 (2020) BindingDB Entry DOI: 10.7270/Q2J67KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 106 total ) | Next | Last >> |