

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Bacillus licheniformis) | BDBM50076680 (CHEMBL6461 | Ro-48-1220 | Sodium; (2S,3R,5R)-3-((Z...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Beta-lactamase from Bacillus licheniformis 749/C | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus licheniformis) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Beta-lactamase from Bacillus licheniformis 749/C | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus licheniformis) | BDBM50053180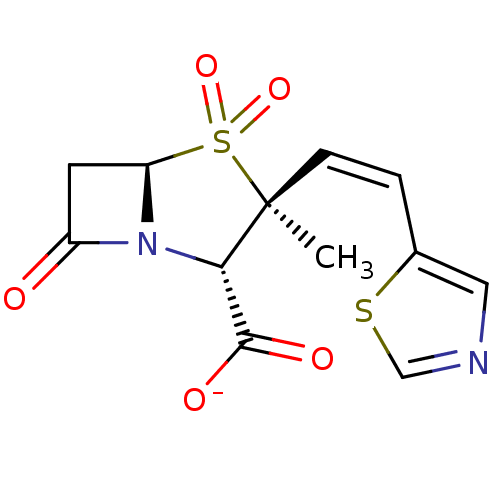 (CHEMBL332565 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Bacillus licheniformis 749/C ,class A of Beta-lactamase | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus licheniformis) | BDBM50053183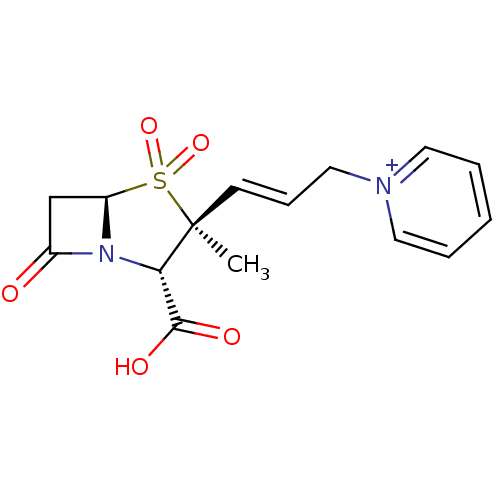 (CHEMBL123481 | Trifluoro-methanesulfonate1-[(E)-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Bacillus licheniformis 749/C ,class A of Beta-lactamase | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus licheniformis) | BDBM50053179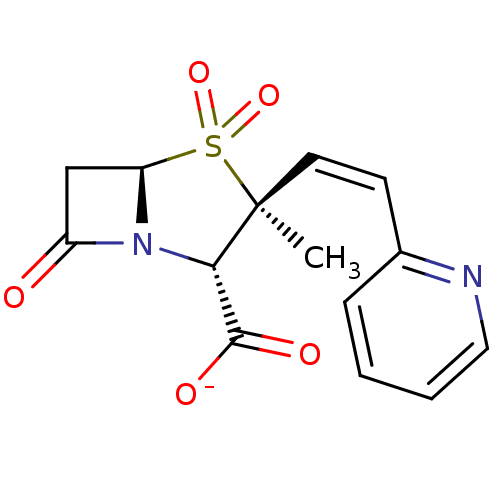 (CHEMBL121924 | Sodium; (2S,3S,5R)-3-methyl-4,4,7-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Bacillus licheniformis 749/C ,class A of Beta-lactamase | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus licheniformis) | BDBM50053181 ((2S,3S,5R)-3-((Z)-2-Ethoxycarbonyl-vinyl)-3-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Bacillus licheniformis 749/C ,class A of Beta-lactamase | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus licheniformis) | BDBM50053175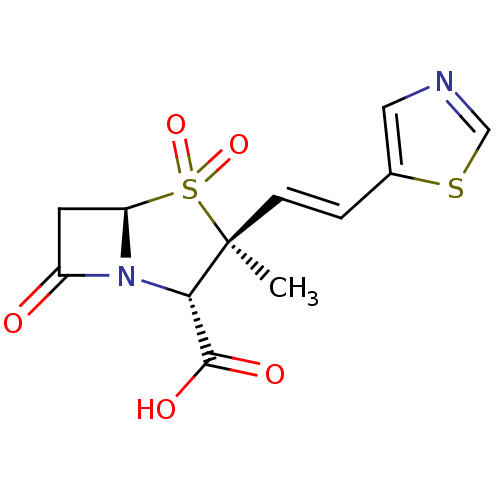 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-((E)-2-thiazol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-LaRoche Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of Bacillus licheniformis 749/C ,class A of Beta-lactamase | J Med Chem 39: 3712-22 (1996) Article DOI: 10.1021/jm9601967 BindingDB Entry DOI: 10.7270/Q2ZK5H99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||