

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50337250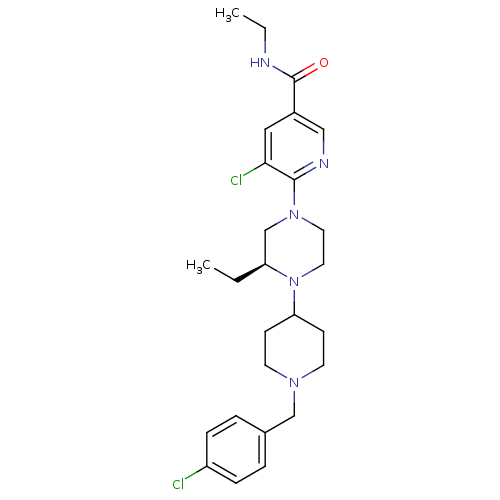 ((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at rat CXCR3 | Bioorg Med Chem Lett 21: 1527-31 (2011) Article DOI: 10.1016/j.bmcl.2010.12.114 BindingDB Entry DOI: 10.7270/Q2CZ37FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50337251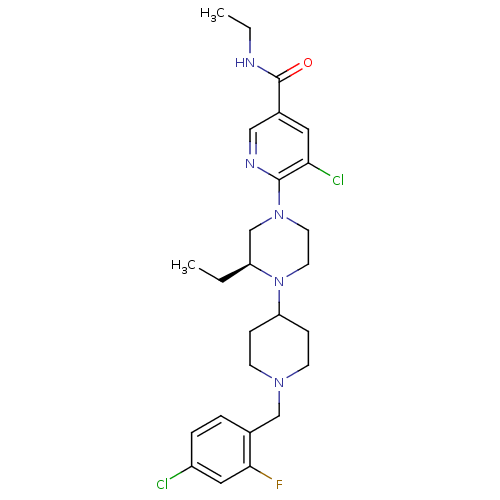 ((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at rat CXCR3 | Bioorg Med Chem Lett 21: 1527-31 (2011) Article DOI: 10.1016/j.bmcl.2010.12.114 BindingDB Entry DOI: 10.7270/Q2CZ37FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50310487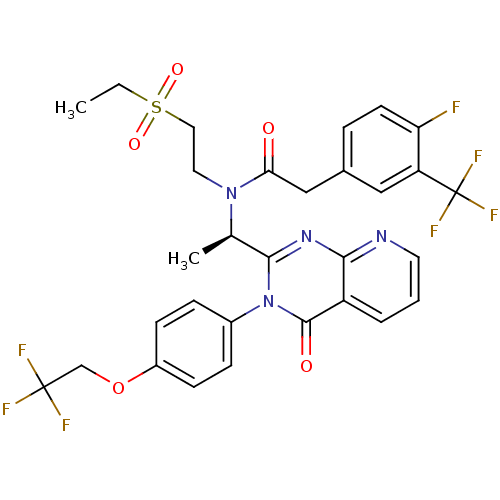 ((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-IP10 from rat CXCR3 expressed in human PBMC after 2 hrs in RPMI buffer by scintillation counting | Bioorg Med Chem Lett 19: 5114-8 (2009) Article DOI: 10.1016/j.bmcl.2009.07.032 BindingDB Entry DOI: 10.7270/Q2FX79KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349673 (CHEMBL1809039) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Ex vivo receptor occupancy of CXCR3 in rat blood assessed as inhibition of ITAC binding after 1 hr by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349672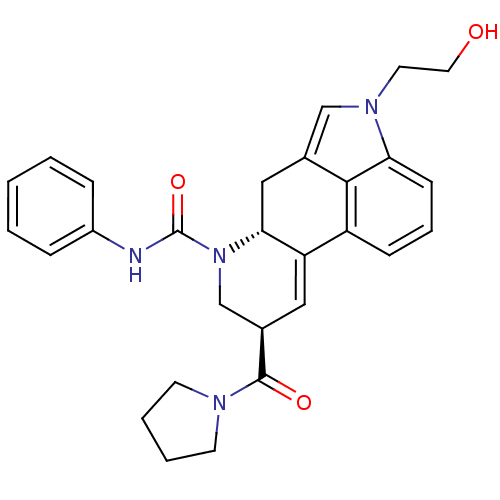 (CHEMBL1809038) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Ex vivo receptor occupancy of CXCR3 in rat blood assessed as inhibition of ITAC binding after 1 hr by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50337218 ((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at rat CXCR3 | Bioorg Med Chem Lett 21: 1527-31 (2011) Article DOI: 10.1016/j.bmcl.2010.12.114 BindingDB Entry DOI: 10.7270/Q2CZ37FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349679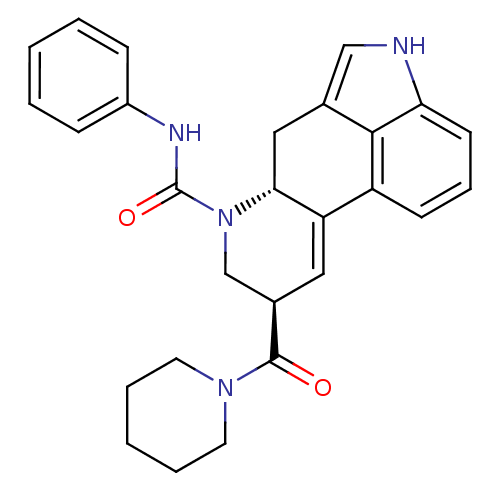 (CHEMBL1809000) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at CXCR3 in rat leukocytes assessed as inhibition of ITAC-induced cell migration by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349674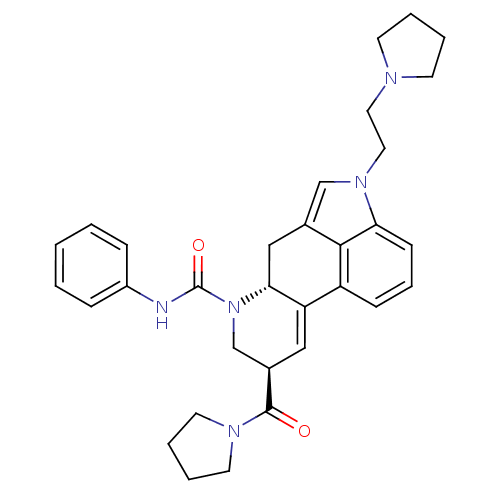 (CHEMBL1809040) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Ex vivo receptor occupancy of CXCR3 in rat blood assessed as inhibition of ITAC binding after 1 hr by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349675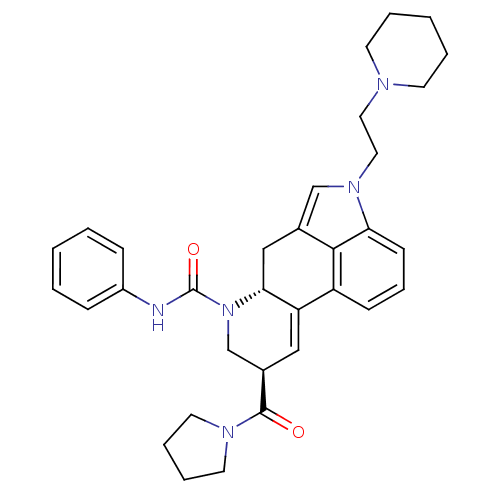 (CHEMBL1809041) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Ex vivo receptor occupancy of CXCR3 in rat blood assessed as inhibition of ITAC binding after 1 hr by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349678 (CHEMBL1806523) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at CXCR3 in rat leukocytes assessed as inhibition of ITAC-induced cell migration by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349676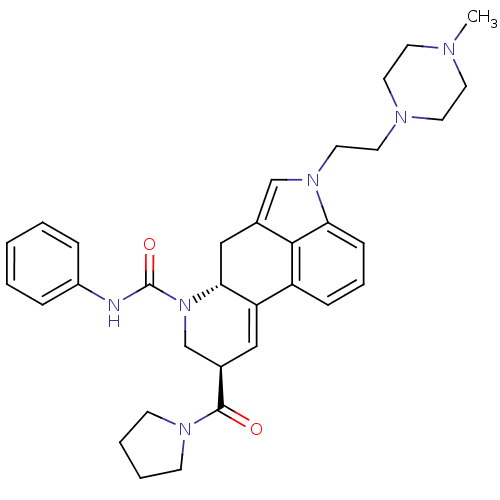 (CHEMBL1809042) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Ex vivo receptor occupancy of CXCR3 in rat blood assessed as inhibition of ITAC binding after 1 hr by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349677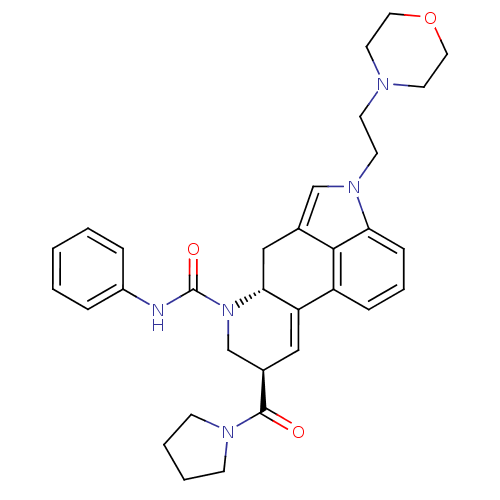 (CHEMBL1809043) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Ex vivo receptor occupancy of CXCR3 in rat blood assessed as inhibition of ITAC binding after 1 hr by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50299165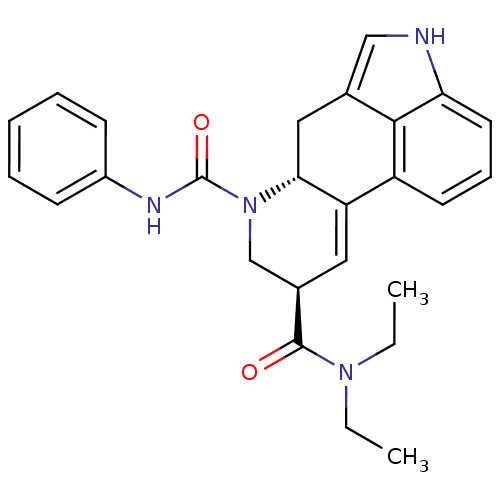 ((6aR,9R)-N9,N9-diethyl-N7-phenyl-6,6a,8,9-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at CXCR3 in rat leukocytes assessed as inhibition of ITAC-induced cell migration by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349649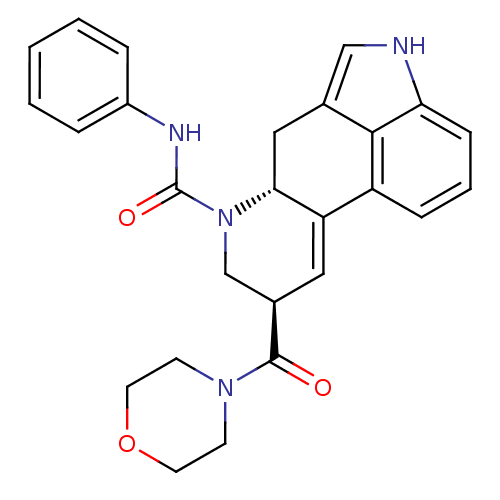 (CHEMBL1809008) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at CXCR3 in rat leukocytes assessed as inhibition of ITAC-induced cell migration by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50337217 (5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonist activity at rat CXCR3 | Bioorg Med Chem Lett 21: 1527-31 (2011) Article DOI: 10.1016/j.bmcl.2010.12.114 BindingDB Entry DOI: 10.7270/Q2CZ37FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349652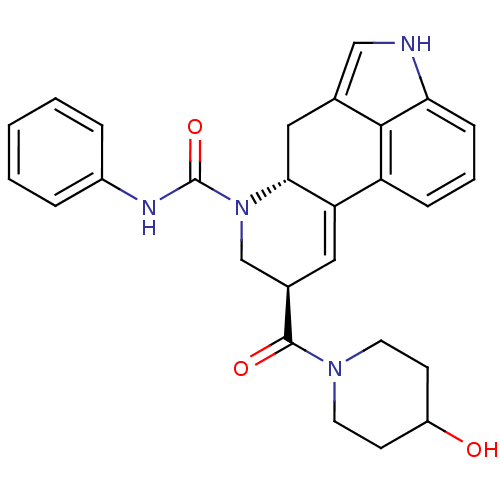 (CHEMBL1809011) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Ex vivo receptor occupancy of CXCR3 in rat blood assessed as inhibition of ITAC binding after 1 hr by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Rattus norvegicus) | BDBM50349678 (CHEMBL1806523) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Ex vivo receptor occupancy of CXCR3 in rat blood assessed as inhibition of ITAC binding after 1 hr by flow cytometry | Bioorg Med Chem Lett 21: 4745-9 (2011) Article DOI: 10.1016/j.bmcl.2011.06.070 BindingDB Entry DOI: 10.7270/Q2VM4CN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||