

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM525308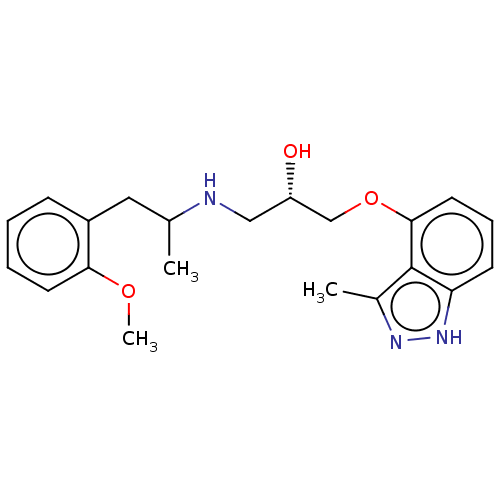 (US11173144, Compound 72) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ADRB2 IC50 from binding assay(nM), Determined using the Tag-lite Adrenoceptor Beta2 receptor ligand binding assay. | Citation and Details BindingDB Entry DOI: 10.7270/Q2D50R4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM525317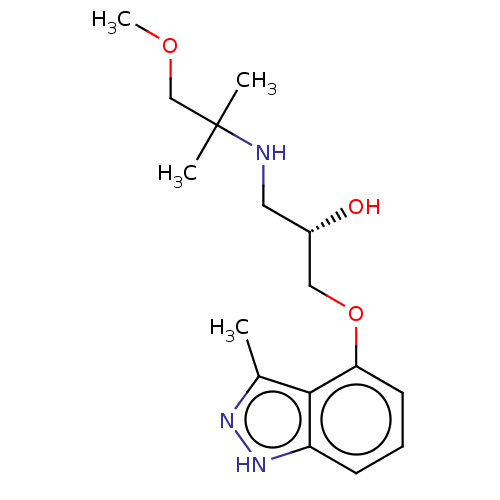 (US11173144, Compound 90) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ADRB2 IC50 from binding assay(nM), Determined using the Tag-lite Adrenoceptor Beta2 receptor ligand binding assay. | Citation and Details BindingDB Entry DOI: 10.7270/Q2D50R4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM525320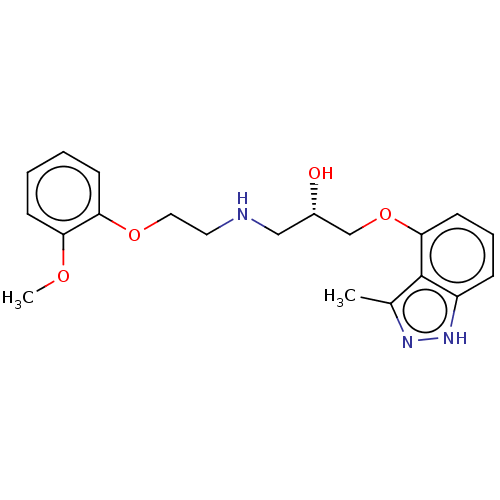 (US11173144, Compound 52) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ADRB2 IC50 from binding assay(nM), Determined using the Tag-lite Adrenoceptor Beta2 receptor ligand binding assay. | Citation and Details BindingDB Entry DOI: 10.7270/Q2D50R4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM525316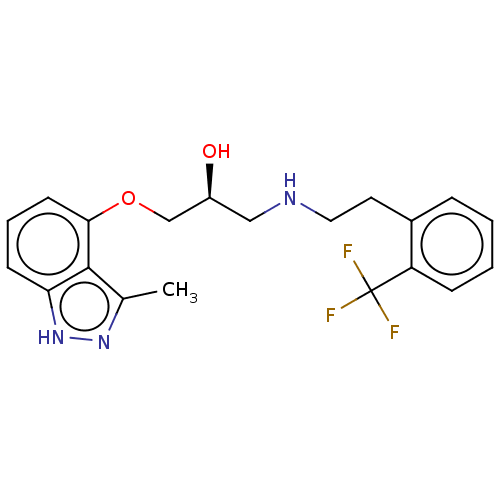 (US11173144, Compound 69) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ADRB2 IC50 from binding assay(nM), Determined using the Tag-lite Adrenoceptor Beta2 receptor ligand binding assay. | Citation and Details BindingDB Entry DOI: 10.7270/Q2D50R4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM525318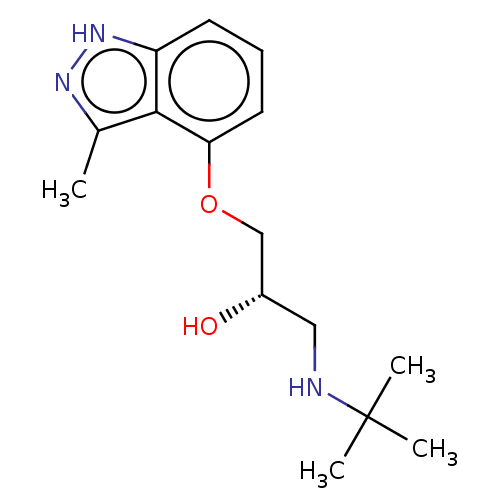 (US11173144, Compound 75) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ADRB2 IC50 from binding assay(nM), Determined using the Tag-lite Adrenoceptor Beta2 receptor ligand binding assay. | Citation and Details BindingDB Entry DOI: 10.7270/Q2D50R4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM525322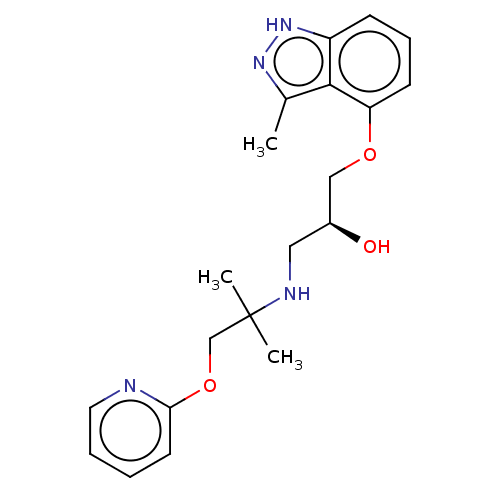 (US11173144, Compound 86) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.144 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ADRB2 IC50 from binding assay(nM), Determined using the Tag-lite Adrenoceptor Beta2 receptor ligand binding assay. | Citation and Details BindingDB Entry DOI: 10.7270/Q2D50R4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM525319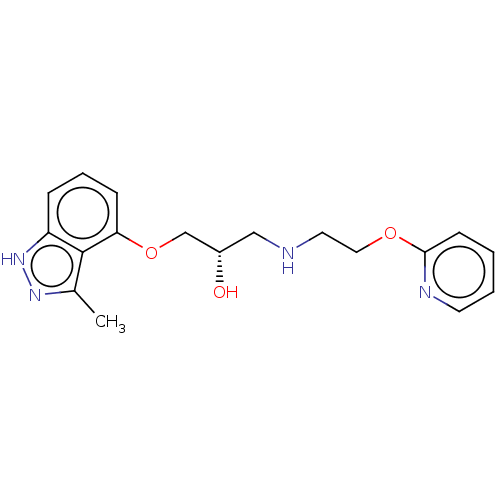 (US11173144, Compound 87) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ADRB2 IC50 from binding assay(nM), Determined using the Tag-lite Adrenoceptor Beta2 receptor ligand binding assay. | Citation and Details BindingDB Entry DOI: 10.7270/Q2D50R4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM525321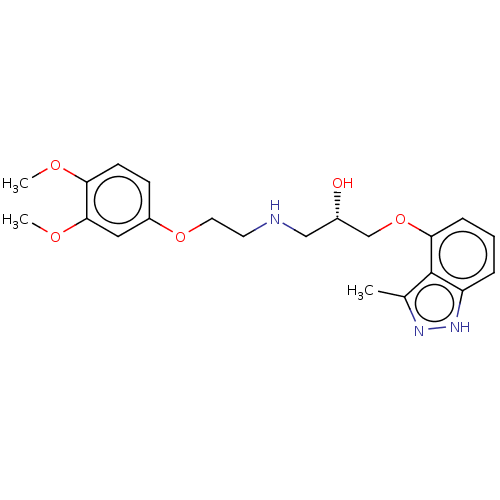 (US11173144, Compound 55) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ADRB2 IC50 from binding assay(nM), Determined using the Tag-lite Adrenoceptor Beta2 receptor ligand binding assay. | Citation and Details BindingDB Entry DOI: 10.7270/Q2D50R4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257340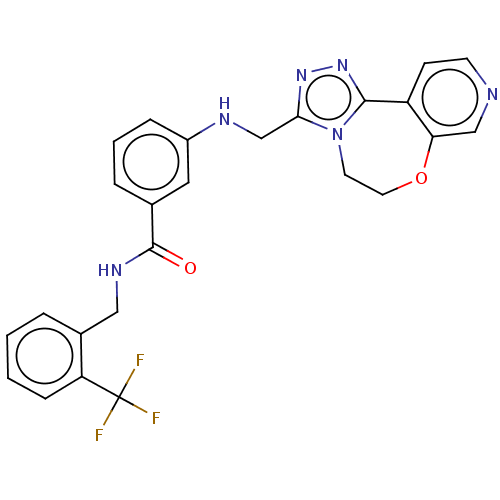 (CHEMBL4072828) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257328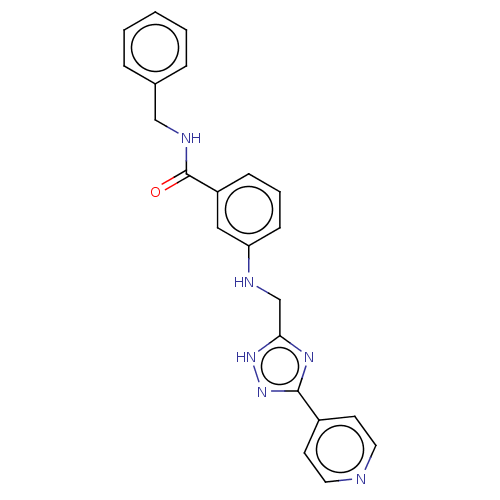 (CHEMBL4082775) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257365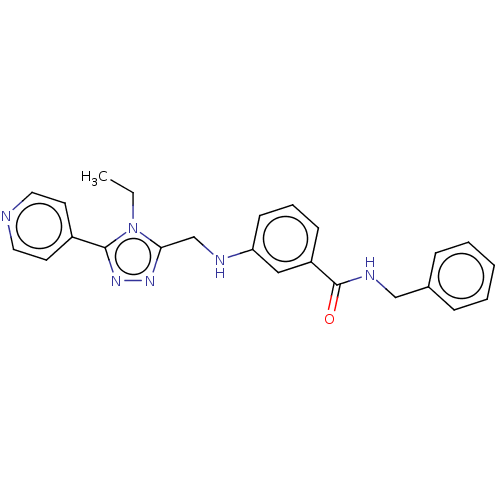 (CHEMBL4083276) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257344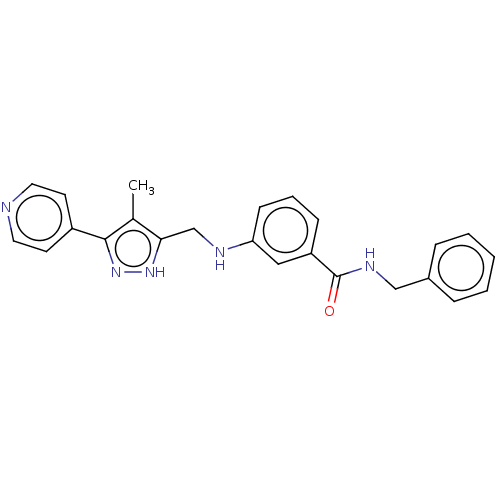 (CHEMBL4065690) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257350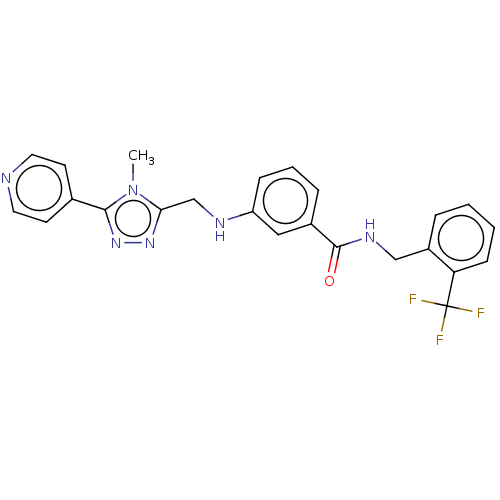 (CHEMBL1738877) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257350 (CHEMBL1738877) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01511 BindingDB Entry DOI: 10.7270/Q22Z19M2 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50257351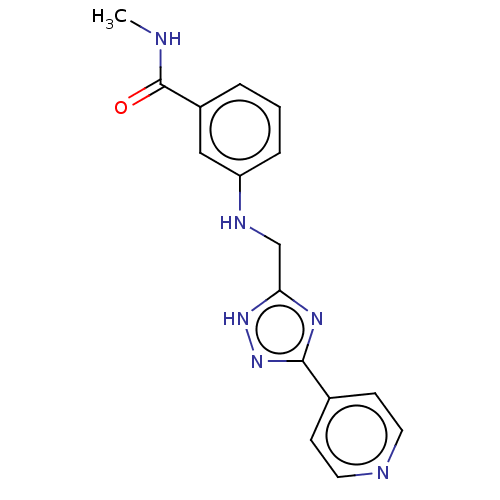 (CHEMBL4095595) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Shonan Research Center, Pharmaceutical Research Division, Takeda Pharmaceutical Co., Ltd. , 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan. Curated by ChEMBL | Assay Description Inhibition of recombinant human full length GST-tagged GRK3 expressed in baculovirus using ulight topo2alpha as substrate preincubated for 60 mins fo... | J Med Chem 60: 6942-6990 (2017) Article DOI: 10.1021/acs.jmedchem.7b00443 BindingDB Entry DOI: 10.7270/Q280552Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM3149 (2-{[2,6-dihydroxy-4-({[(3R,4R)-3-[(4-hydroxybenzen...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of GRK3-mediated bovine tubulin phosphorylation by scintillation counting | J Med Chem 53: 1867-70 (2010) Article DOI: 10.1021/jm9017515 BindingDB Entry DOI: 10.7270/Q2P26Z8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human GRK3 using casein as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 861 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GRK3 using casein as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50566947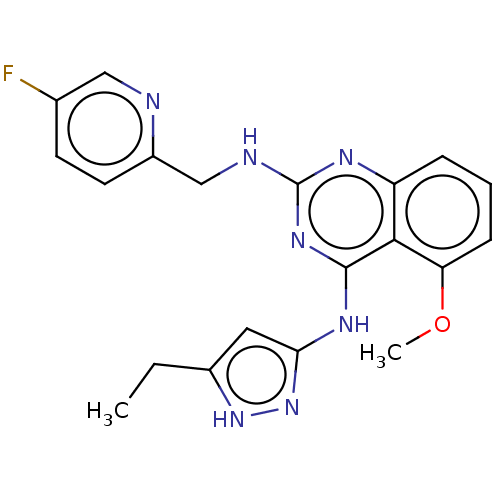 (CHEMBL4847703) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GRK3 using casein as substrate in presence of [gamma33P]ATP by radiometric hotspot kinase assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00506 BindingDB Entry DOI: 10.7270/Q20K2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50566946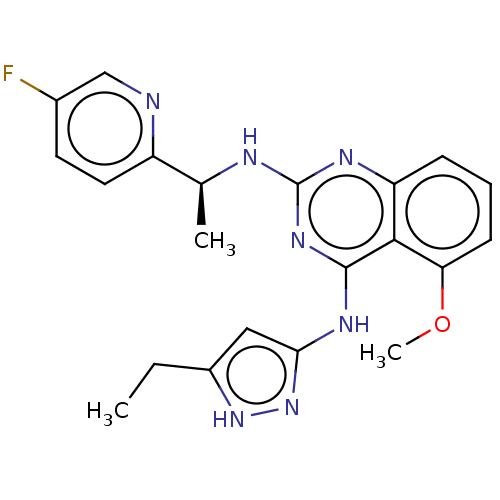 (CHEMBL4854871) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GRK3 using casein as substrate in presence of [gamma33P]ATP by radiometric hotspot kinase assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00506 BindingDB Entry DOI: 10.7270/Q20K2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50519662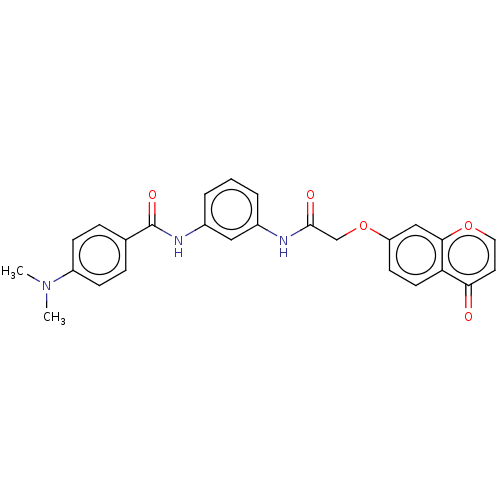 (CHEMBL4438748) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant full length human GRK3 using casein as substrate after 40 mins in presence of [gamma-33ATP] by radiometric scintillation co... | J Med Chem 62: 10691-10710 (2019) Article DOI: 10.1021/acs.jmedchem.9b01143 BindingDB Entry DOI: 10.7270/Q2MC93FG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50135286 (CHEMBL3745885) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human GRK3 using casein as substrate | Bioorg Med Chem 24: 521-44 (2016) Article DOI: 10.1016/j.bmc.2015.11.045 BindingDB Entry DOI: 10.7270/Q24Q7WT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50566949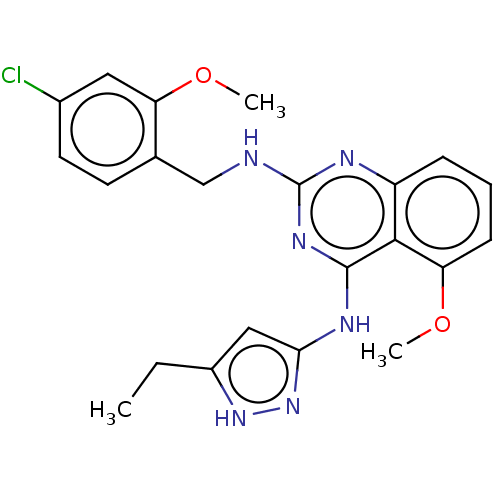 (CHEMBL4877302) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GRK3 using casein as substrate in presence of [gamma33P]ATP by radiometric hotspot kinase assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00506 BindingDB Entry DOI: 10.7270/Q20K2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-adrenergic receptor kinase 2 (Homo sapiens (Human)) | BDBM50537742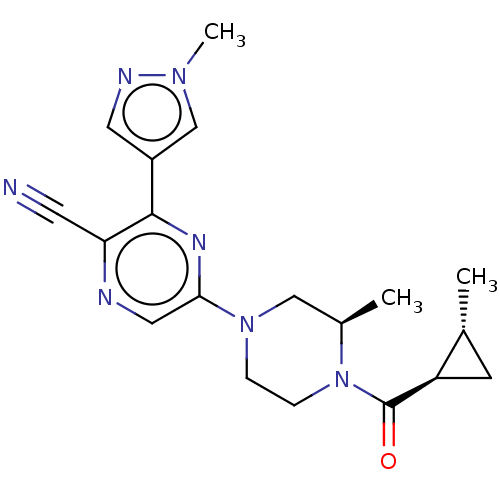 (CHEMBL4634634 | US11179389, Compound 1-14) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of recombinant full length human GST-tagged GRK3 expressed in baculovirus expression system using serine/threonine-16 peptide as substrate... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126715 BindingDB Entry DOI: 10.7270/Q2HM5CZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||