

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50555514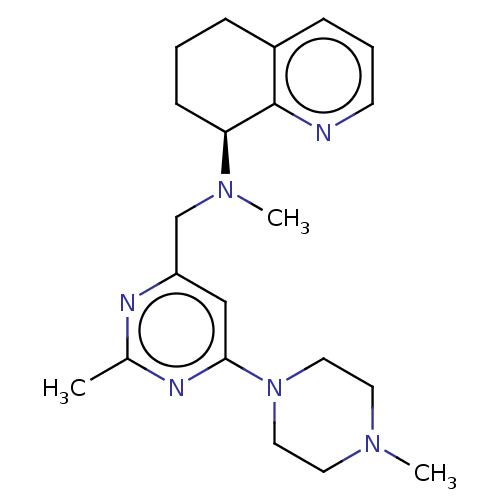 (CHEMBL4751485) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 in human CD4-positive T cells assessed as inhibition of CXCL12-induced calcium signal incubated for 20 mins by FLIPR ass... | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111914 BindingDB Entry DOI: 10.7270/Q2Q81HRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50565499 (CHEMBL4799439) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 in human CD4-positive T cells assessed as inhibition of CXCL12-induced cytosolic calcium flux preincubated for 20 mins f... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112537 BindingDB Entry DOI: 10.7270/Q26M3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50225415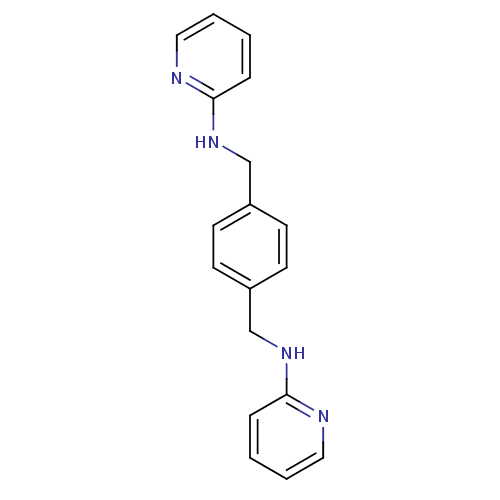 (CHEMBL237830 | N,N'-di-2-pyridinyl-1,4-benzenedime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Calabria Curated by ChEMBL | Assay Description Binding affinity to CXCR4 in human MDA-MB-231 cells preincubated for 15 mins followed by biotinylated TN41003 addition measured after 30 mins by rhod... | Eur J Med Chem 139: 519-530 (2017) Article DOI: 10.1016/j.ejmech.2017.08.027 BindingDB Entry DOI: 10.7270/Q21J9D9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Inhibition of CXCR4-mediated chemotaxis in SDF1-stimulated human U937 cells treated 15 mins before SDF1 challenge measured after 2 hrs by luminescenc... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50565506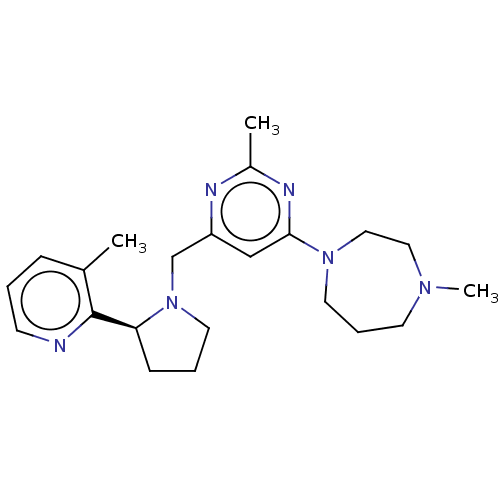 (CHEMBL4792975) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 in human CD4-positive T cells assessed as inhibition of CXCL12-induced cytosolic calcium flux preincubated for 20 mins f... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112537 BindingDB Entry DOI: 10.7270/Q26M3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50225416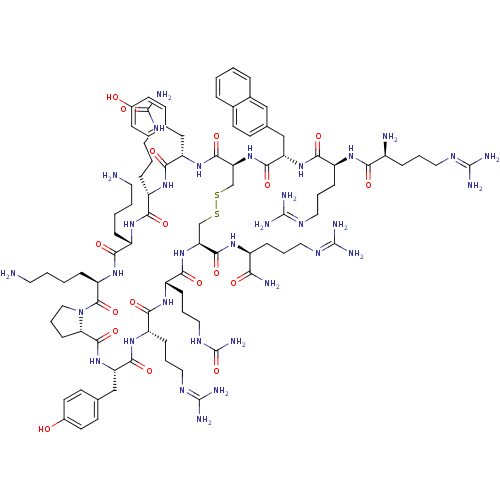 (CHEMBL393882 | TN-14003) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of CXCR4 in MDA-MB-231 cells | J Med Chem 50: 5655-64 (2007) Article DOI: 10.1021/jm070679i BindingDB Entry DOI: 10.7270/Q2X066RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50335557 (CHEMBL1652605 | N,N-dipropyl-N'-[4-({[(1H-imidazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 (unknown origin) expressed in CHO cells by scintillation counting analysis | Eur J Med Chem 149: 148-169 (2018) Article DOI: 10.1016/j.ejmech.2018.02.043 BindingDB Entry DOI: 10.7270/Q2F76G5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50335557 (CHEMBL1652605 | N,N-dipropyl-N'-[4-({[(1H-imidazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Infectious Diseases Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha form CXCR4 expressed in CHO cells by scintillation counting | Antimicrob Agents Chemother 53: 2940-8 (2009) Article DOI: 10.1128/AAC.01727-08 BindingDB Entry DOI: 10.7270/Q2GF0TR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458238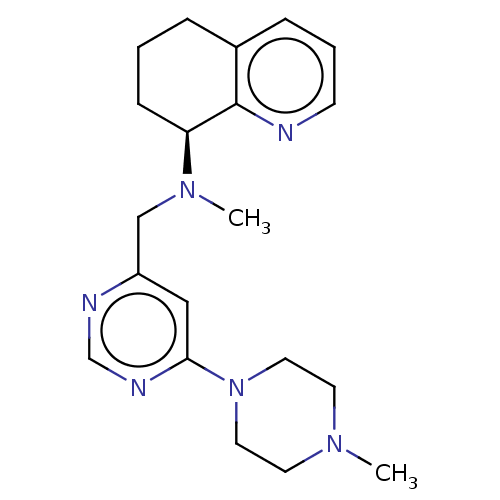 (CHEMBL4203703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CD4+ T cells assessed as inhibition of CXCL12-mediated cytosolic calcium level preincubated with compounds foll... | Eur J Med Chem 149: 30-44 (2018) Article DOI: 10.1016/j.ejmech.2018.02.042 BindingDB Entry DOI: 10.7270/Q2TH8QBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50347490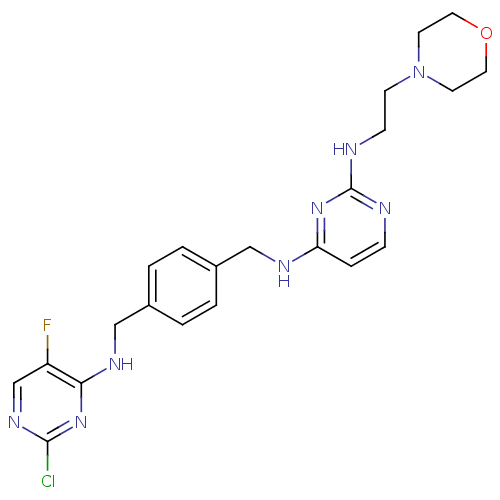 (CHEMBL1802333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo... | J Med Chem 53: 8556-68 (2010) Article DOI: 10.1021/jm100786g BindingDB Entry DOI: 10.7270/Q2PK0GH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035696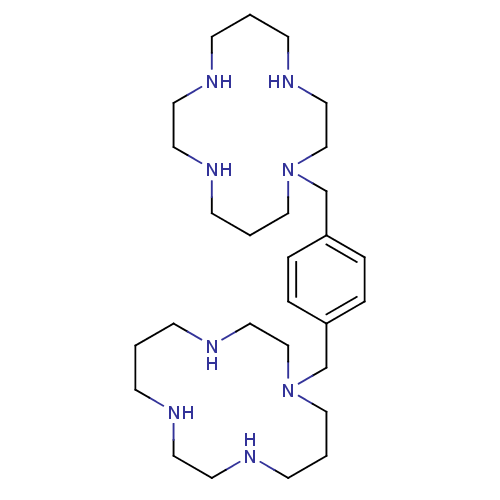 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Displacement of [125I]SDF1alpha from CXCR4 (unknown origin) expressed in CHOK1 cells | J Biol Chem 282: 30062-9 (2007) Article DOI: 10.1074/jbc.M705302200 BindingDB Entry DOI: 10.7270/Q27M07Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated 30 mins before agonist challenge... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443545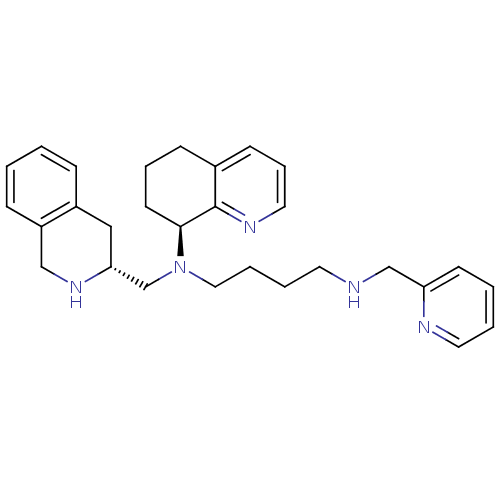 (CHEMBL3091683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM221860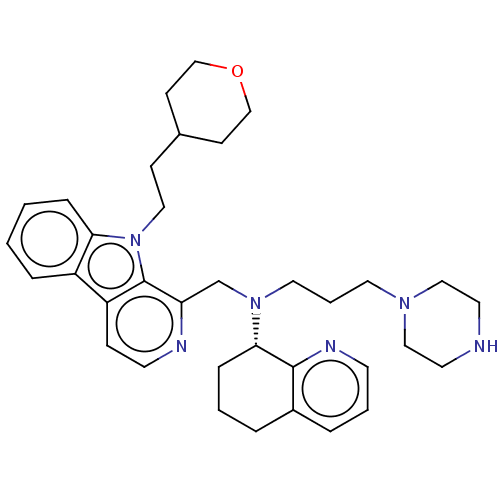 (US9314468, Table 7, Compound 147) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Altiris Therapeutics, Inc. US Patent | Assay Description Functional modulation of CXCR4 was determined by calcium mobilization assay using leukemic lymphoid CEM cells, which naturally express high levels of... | US Patent US9314468 (2016) BindingDB Entry DOI: 10.7270/Q2DF6Q2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM221760 (US9314468, Table 7, Compound 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Altiris Therapeutics, Inc. US Patent | Assay Description Functional modulation of CXCR4 was determined by calcium mobilization assay using leukemic lymphoid CEM cells, which naturally express high levels of... | US Patent US9314468 (2016) BindingDB Entry DOI: 10.7270/Q2DF6Q2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50369468 (CHEMBL1202231) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AnorMED Inc. Curated by ChEMBL | Assay Description Inhibitory activity against CX3C chemokine receptor 4-specific monoclonal antibody 12G5 (mAb-12G5) binding to human chemokinin receptor CXCR4 in lymp... | J Med Chem 42: 3971-81 (1999) BindingDB Entry DOI: 10.7270/Q2K0750M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50277349 (CHEMBL4163246) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Calabria Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 (unknown origin) | Eur J Med Chem 139: 519-530 (2017) Article DOI: 10.1016/j.ejmech.2017.08.027 BindingDB Entry DOI: 10.7270/Q21J9D9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated before agonist challenge measure... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50565504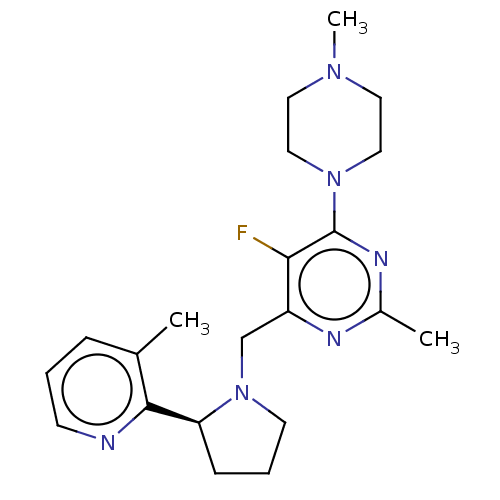 (CHEMBL4799191) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 in human CD4-positive T cells assessed as inhibition of CXCL12-induced cytosolic calcium flux preincubated for 20 mins f... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112537 BindingDB Entry DOI: 10.7270/Q26M3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247128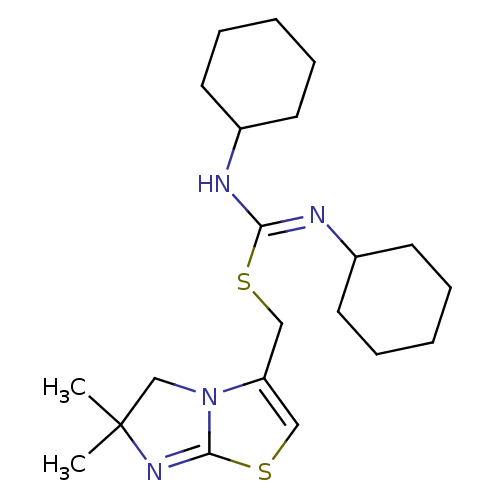 ((6,6-dimethyl-5,6-dihydroimidazo[2,1-b][1,3]thiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50333890 (CHEMBL1644092 | N-((1H-benzo[d]imidazol-2-yl)methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells expressing CD4 assessed as inhibition of SDF-1-induced Ca2+ signaling | Bioorg Med Chem Lett 21: 262-6 (2010) Article DOI: 10.1016/j.bmcl.2010.11.023 BindingDB Entry DOI: 10.7270/Q21N81D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50379090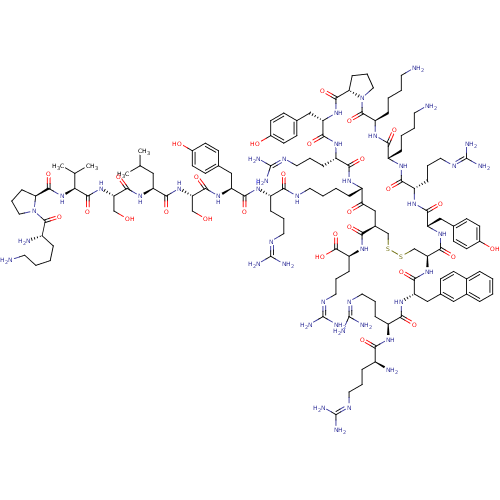 (CHEMBL2012527) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1alpha from human CXCR4 receptor expressed in HEK293 cells after 1 hr by gamma counting | ACS Med Chem Lett 2: 597-602 (2011) Article DOI: 10.1021/ml200084n BindingDB Entry DOI: 10.7270/Q2W096XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50250705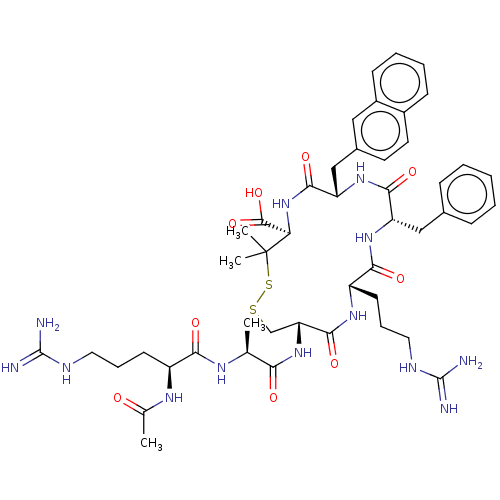 (CHEMBL4078698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Campania Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from CXCR4 in human CCRF-CEM cells after 1 hr by gamma counting method | J Med Chem 60: 9641-9652 (2017) Article DOI: 10.1021/acs.jmedchem.7b01062 BindingDB Entry DOI: 10.7270/Q28S4SBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50379088 (CHEMBL2012525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1alpha from human CXCR4 receptor expressed in HEK293 cells after 1 hr by gamma counting | ACS Med Chem Lett 2: 597-602 (2011) Article DOI: 10.1021/ml200084n BindingDB Entry DOI: 10.7270/Q2W096XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50517802 (CHEMBL4457992) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU) Curated by ChEMBL | Assay Description Competitive inhibition of TAMRAAc-TZ14011 binding to CXCR4 (unknown origin) expressed in CHO cells in presence of ZnCl2 by NanoBRET assay relative to... | Bioorg Med Chem 27: 1130-1138 (2019) Article DOI: 10.1016/j.bmc.2019.02.013 BindingDB Entry DOI: 10.7270/Q2QR51HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50603567 (CHEMBL5172755) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114150 BindingDB Entry DOI: 10.7270/Q26W9G5W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50225415 (CHEMBL237830 | N,N'-di-2-pyridinyl-1,4-benzenedime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated fo... | J Med Chem 53: 8556-68 (2010) Article DOI: 10.1021/jm100786g BindingDB Entry DOI: 10.7270/Q2PK0GH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50565505 (CHEMBL4777386) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 in human CD4-positive T cells assessed as inhibition of CXCL12-induced cytosolic calcium flux preincubated for 20 mins f... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112537 BindingDB Entry DOI: 10.7270/Q26M3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50363974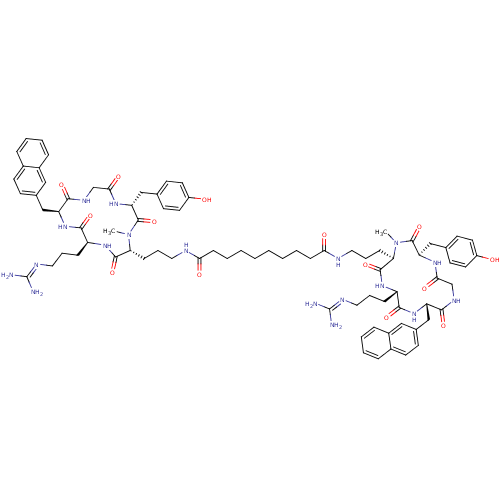 (CHEMBL1949739) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [125I]-CPCR4 from CXCR4 receptor in human Jurkat cells after 2 hrs by gamma counting | J Med Chem 54: 7648-62 (2011) Article DOI: 10.1021/jm2009716 BindingDB Entry DOI: 10.7270/Q23T9HQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125954 (CHEMBL3627858) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125950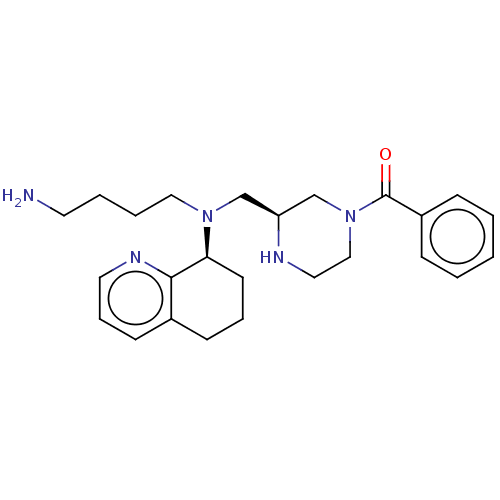 (CHEMBL3627793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50363984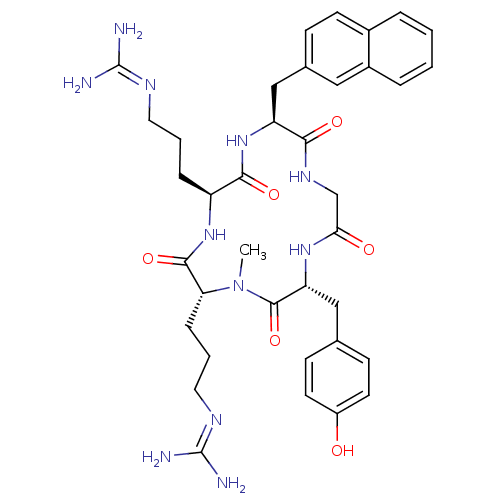 (CHEMBL1949730) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [125I]-CPCR4 from CXCR4 receptor in human Jurkat cells after 2 hrs by gamma counting | J Med Chem 54: 7648-62 (2011) Article DOI: 10.1021/jm2009716 BindingDB Entry DOI: 10.7270/Q23T9HQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125958 (CHEMBL3627862) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Mus musculus) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at mouse CXCR4 expressed in human U2OS cells assessed as inhibition of SDF1-induced increase in intracellular calcium level by FL... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247129 (1,3-dicycloheptyl-2-((6,6-dimethyl-5,6-dihydroimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50379089 (CHEMBL2012526) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1alpha from human CXCR4 receptor expressed in HEK293 cells after 1 hr by gamma counting | ACS Med Chem Lett 2: 597-602 (2011) Article DOI: 10.1021/ml200084n BindingDB Entry DOI: 10.7270/Q2W096XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50458252 (CHEMBL4214960) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CD4+ T cells assessed as inhibition of CXCL12-mediated cytosolic calcium level preincubated with compounds foll... | Eur J Med Chem 149: 30-44 (2018) Article DOI: 10.1016/j.ejmech.2018.02.042 BindingDB Entry DOI: 10.7270/Q2TH8QBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.41 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced increase in intracellular calcium level treated 1... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50565501 (CHEMBL4783831) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 in human CD4-positive T cells assessed as inhibition of CXCL12-induced cytosolic calcium flux preincubated for 20 mins f... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112537 BindingDB Entry DOI: 10.7270/Q26M3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50565508 (CHEMBL4789233) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 in human CD4-positive T cells assessed as inhibition of CXCL12-induced cytosolic calcium flux preincubated for 20 mins f... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112537 BindingDB Entry DOI: 10.7270/Q26M3BKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50335557 (CHEMBL1652605 | N,N-dipropyl-N'-[4-({[(1H-imidazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of Mab 12G5 binding to wild type CXCR4 expressed in HEK293 cells | Antimicrob Agents Chemother 53: 2940-8 (2009) Article DOI: 10.1128/AAC.01727-08 BindingDB Entry DOI: 10.7270/Q2GF0TR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035696 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50449948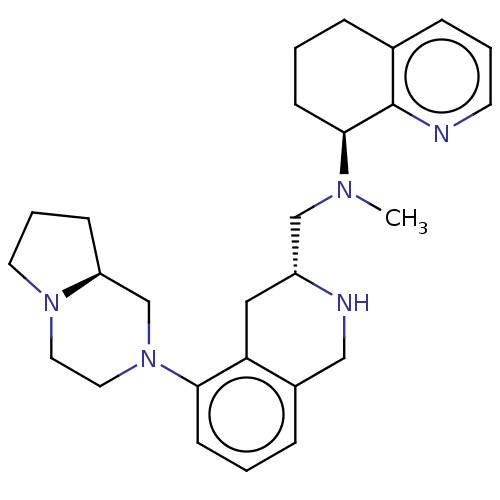 (CHEMBL4173977) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins followed b... | J Med Chem 61: 7168-7188 (2018) Article DOI: 10.1021/acs.jmedchem.8b00450 BindingDB Entry DOI: 10.7270/Q2GH9MJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443543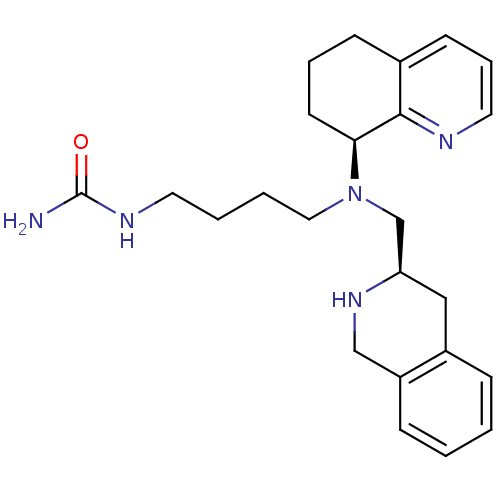 (CHEMBL3091685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50363973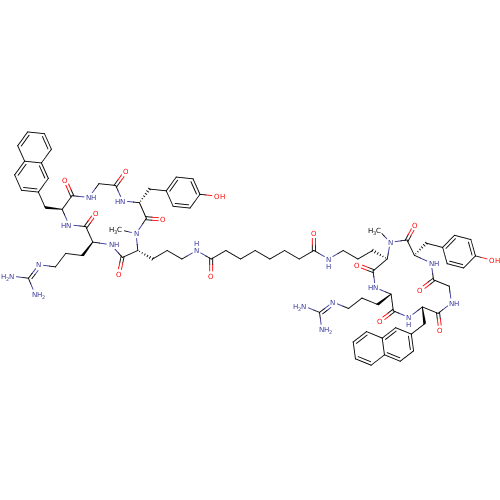 (CHEMBL1949738) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t M£nchen Curated by ChEMBL | Assay Description Displacement of [125I]-CPCR4 from CXCR4 receptor in human Jurkat cells after 2 hrs by gamma counting | J Med Chem 54: 7648-62 (2011) Article DOI: 10.1021/jm2009716 BindingDB Entry DOI: 10.7270/Q23T9HQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202356 (CHEMBL218806 | cyclo(-D-Tyr-D-MeArg-L-Arg-L-Nal-Gl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541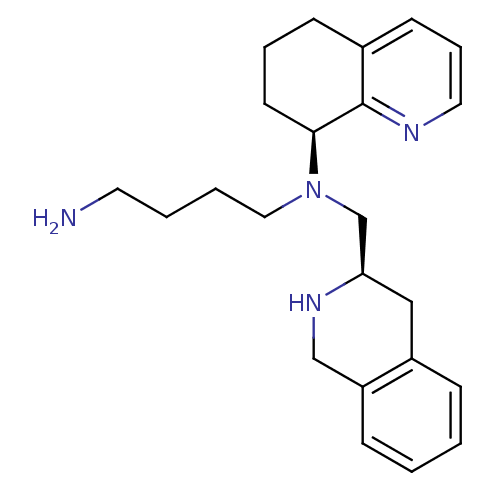 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035696 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114150 BindingDB Entry DOI: 10.7270/Q26W9G5W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50247015 (1,3-dicycloheptyl-2-((5,6-dihydroimidazo[2,1-b]thi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilization | J Med Chem 51: 7915-20 (2008) Article DOI: 10.1021/jm801065q BindingDB Entry DOI: 10.7270/Q2JD4WNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50326057 (([5-(4-Methyl-1-piperazinyl)-2-({methyl[(8S)-5,6,7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Antagonist activity at human CXCR4 expressed in HEK293 cells assessed as inhibition of SDF1-induced response treated before agonist challenge measure... | Antimicrob Agents Chemother 54: 817-24 (2010) Article DOI: 10.1128/AAC.01293-09 BindingDB Entry DOI: 10.7270/Q2F47PBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1332 total ) | Next | Last >> |