 Found 327 hits of ic50 data for polymerid = 50004805
Found 327 hits of ic50 data for polymerid = 50004805 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561047
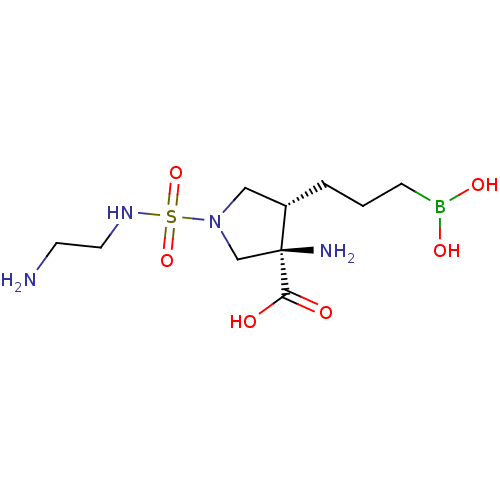
(CHEMBL4764455)Show SMILES NCCNS(=O)(=O)N1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642313
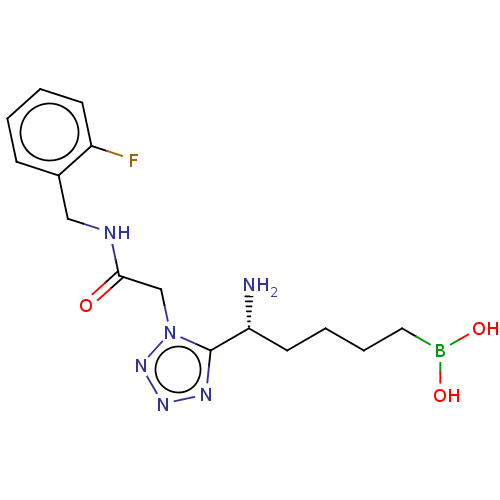
((+)-(R)-(5-amino-5-(1-(2-((2- fluorobenzyl)amino)-...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547931

(CHEMBL4745275) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642315
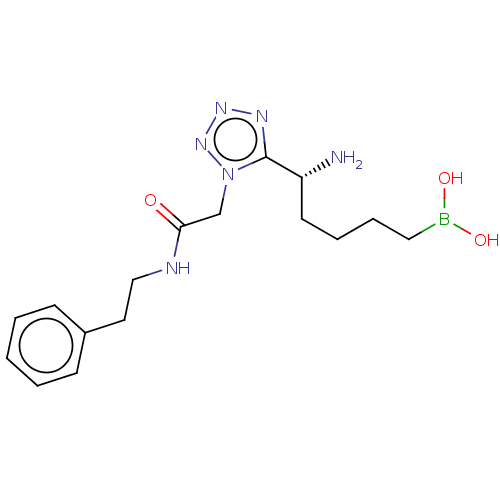
((+)-(R)-(5-amino-5-(1-(2-oxo-2- (phenethylamino)et...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642314
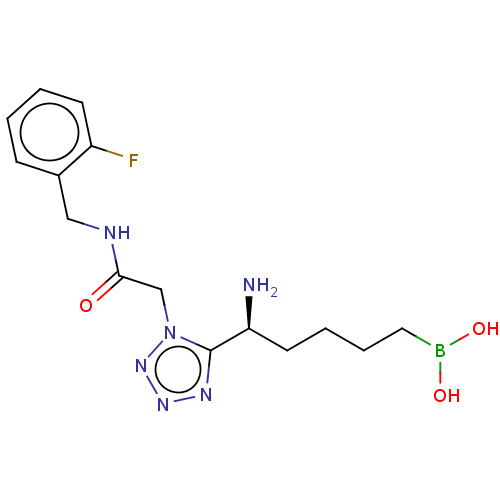
((-)-(S)-(5-amino-5-(1-(2-((2- fluorobenzyl)amino)-...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642312
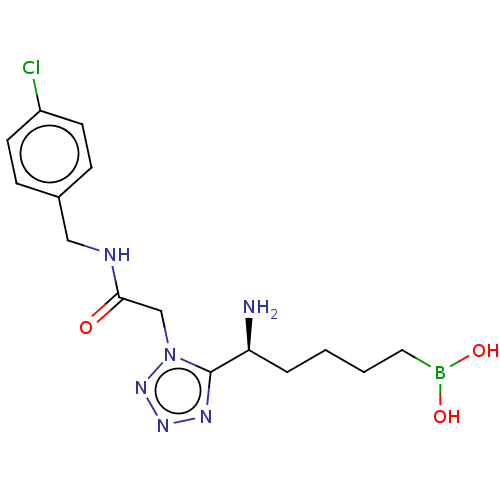
((-)-(S)-(5-amino-5-(1-(2-((4- chlorobenzyl)amino)-...)Show SMILES N[C@@H](CCCCB(O)O)c1nnnn1CC(=O)NCc1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642218
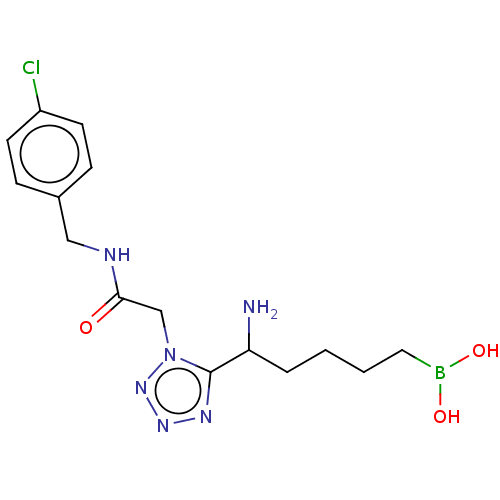
((5-amino-5-(1-(2-((4-chlorobenzyl) amino)-2-oxoeth...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642208
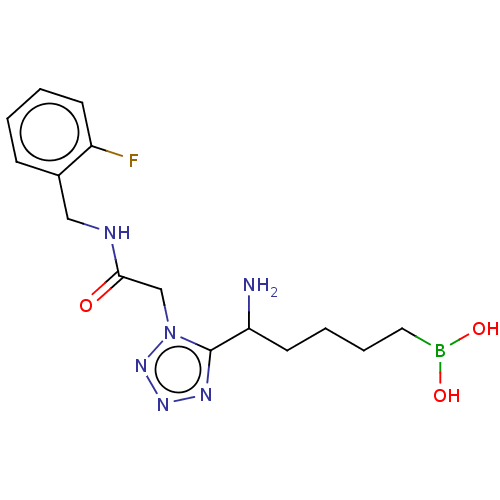
((5-amino-5-(1-(2-((2-fluorobenzyl)amino)- 2-oxoeth...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50350311
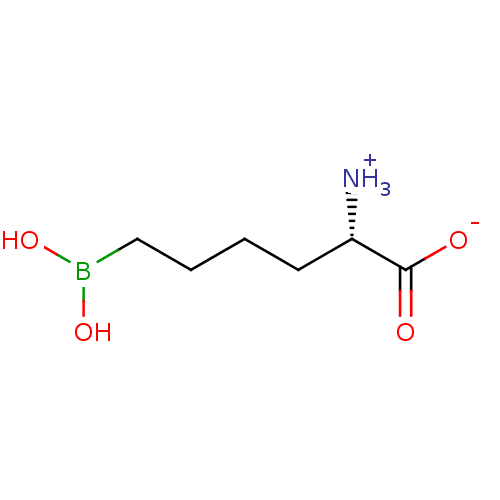
(CHEMBL1812661)Show InChI InChI=1S/C6H14BNO4/c8-5(6(9)10)3-1-2-4-7(11)12/h5,11-12H,1-4,8H2,(H,9,10)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561039

(CHEMBL4749355 | US11420984, Example 23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 by TOGA assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM568474
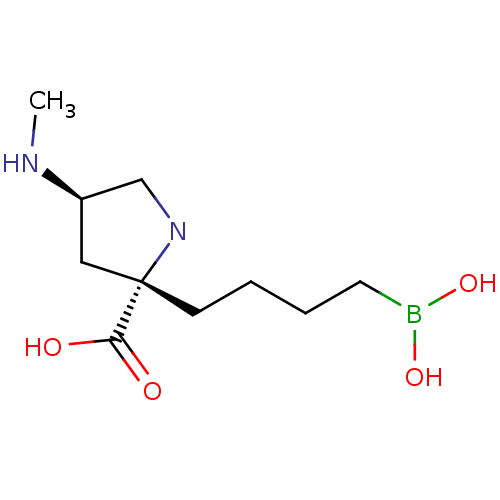
(US11420984, Example 24)Show SMILES CN[C@H]1CN[C@@](CCCCB(O)O)(C1)C(O)=O |r,$;;;;N;;;;;;;;;;;;$| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory effects of Examples 1 to 30 on the activity of Human Arginase 1 and Arginase 2 activity were quantified by measuring the formation of ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q243GV |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642316
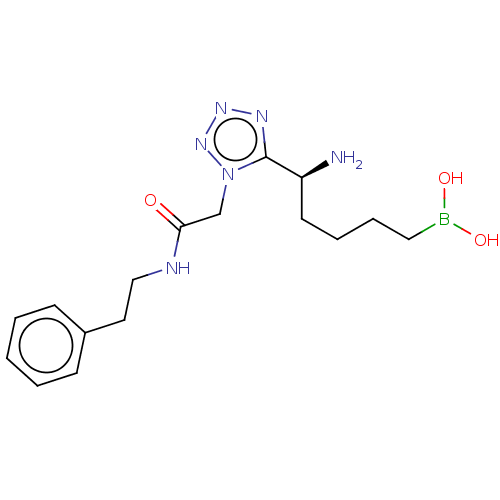
((-)-(S)-(5-amino-5-(1-(2-oxo-2- (phenethylamino)et...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642227
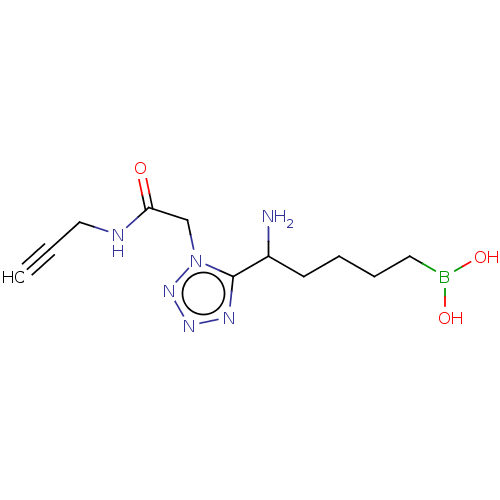
((5-amino-5-(1-(2-oxo-2-(prop-2-yn-1- ylamino)ethyl...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642221
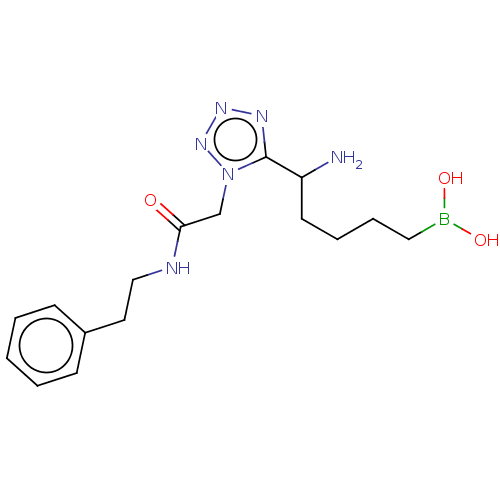
((5-Amino-5-(1-(2-oxo-2- (phenethylamino)ethyl)-1H-...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547934
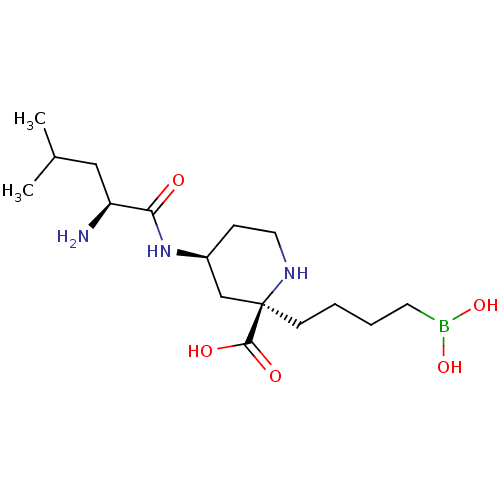
(CHEMBL4757930)Show SMILES CC(C)C[C@H](N)C(=O)N[C@H]1CCN[C@](CCCCB(O)O)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642273
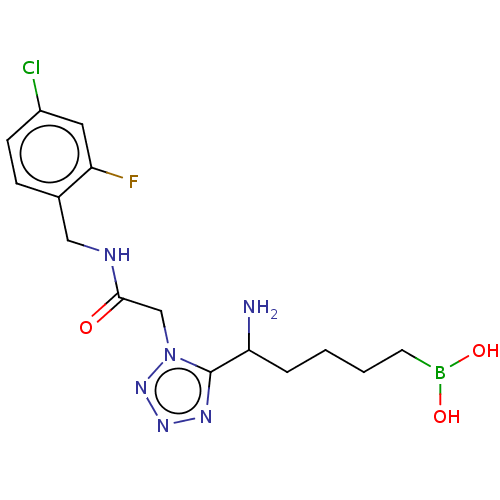
((5-amino-5-(1-(2-((4-chloro-2- fluorobenzyl)amino)...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561038
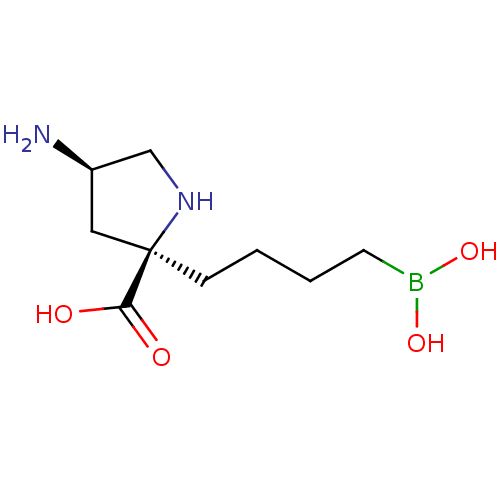
(CHEMBL4752307 | US11420984, Example 7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 by TOGA assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561038

(CHEMBL4752307 | US11420984, Example 7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory effects of Examples 1 to 30 on the activity of Human Arginase 1 and Arginase 2 activity were quantified by measuring the formation of ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Q243GV |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547935
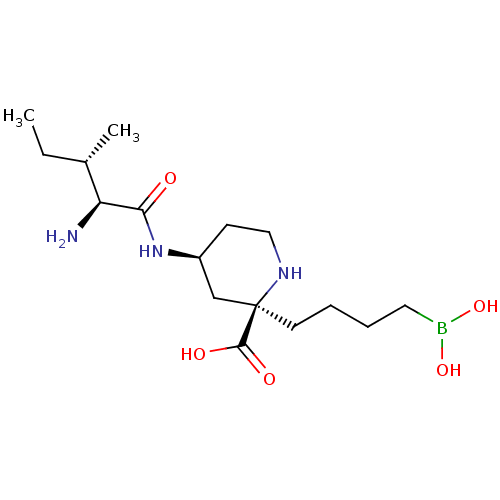
(CHEMBL4753285)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@H]1CCN[C@](CCCCB(O)O)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547933
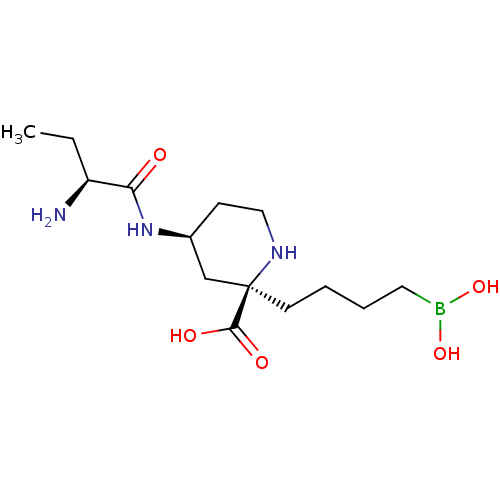
(CHEMBL4752391)Show SMILES CC[C@H](N)C(=O)N[C@H]1CCN[C@](CCCCB(O)O)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
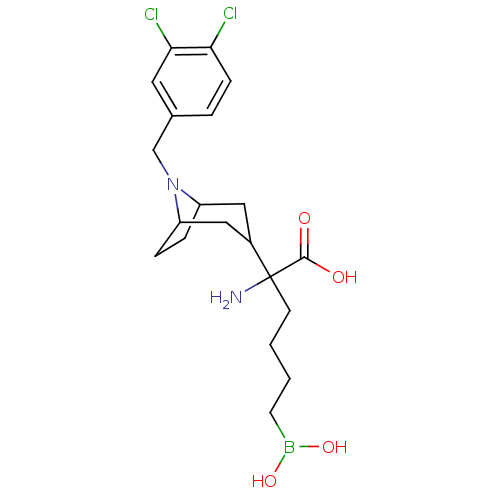
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439247
(CHEMBL2418831)Show SMILES NC(CCCCB(O)O)(C1CC2CCC(C1)N2Cc1ccc(Cl)c(Cl)c1)C(O)=O |TLB:17:16:12.13:9.10.15| Show InChI InChI=1S/C20H29BCl2N2O4/c22-17-6-3-13(9-18(17)23)12-25-15-4-5-16(25)11-14(10-15)20(24,19(26)27)7-1-2-8-21(28)29/h3,6,9,14-16,28-29H,1-2,4-5,7-8,10-12,24H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547936

(CHEMBL4750174)Show SMILES CC(C)(C)[C@H](N)C(=O)N[C@H]1CCN[C@](CCCCB(O)O)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50547932
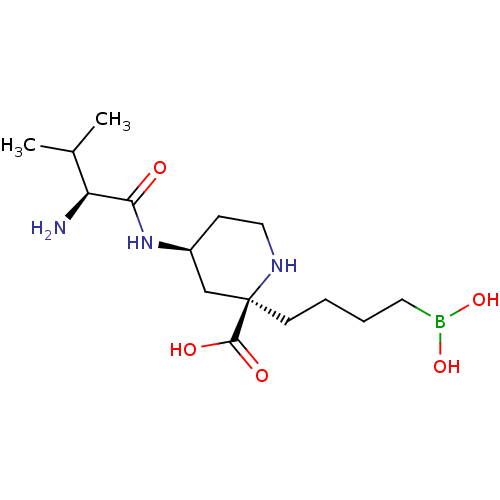
(CHEMBL4778086)Show SMILES CC(C)[C@H](N)C(=O)N[C@H]1CCN[C@](CCCCB(O)O)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant arginase 2 expressed in Escherichia coli using thioarginine by Ellman's assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00593
BindingDB Entry DOI: 10.7270/Q2J106R8 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561034
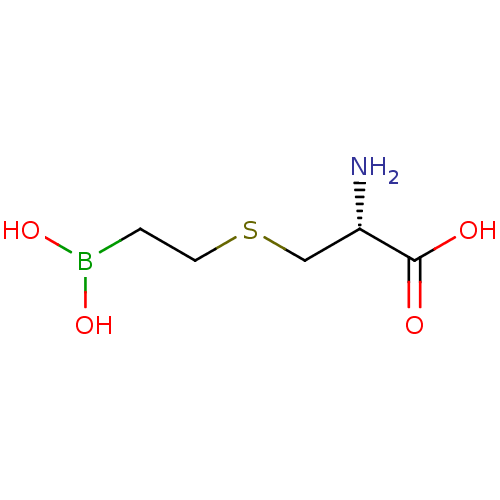
(CHEMBL4244287) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439246

(CHEMBL2418830)Show SMILES NC(CCCCB(O)O)(C1CC2CCC(C1)N2Cc1ccc(Cl)cc1)C(O)=O |TLB:17:16:12.13:9.10.15| Show InChI InChI=1S/C20H30BClN2O4/c22-16-5-3-14(4-6-16)13-24-17-7-8-18(24)12-15(11-17)20(23,19(25)26)9-1-2-10-21(27)28/h3-6,15,17-18,27-28H,1-2,7-13,23H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642309
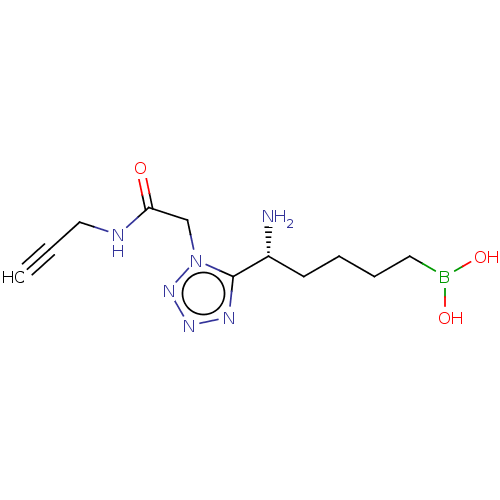
((+)-(R)-(5-amino-5-(1-(2-oxo-2-(prop-2- yn-1-ylami...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439245
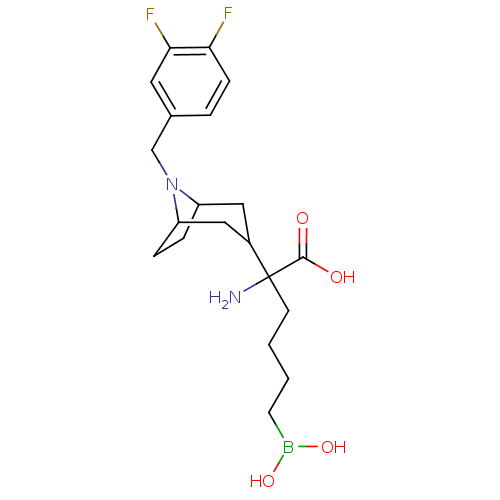
(CHEMBL2418991)Show SMILES NC(CCCCB(O)O)(C1CC2CCC(C1)N2Cc1ccc(F)c(F)c1)C(O)=O |TLB:17:16:12.13:9.10.15| Show InChI InChI=1S/C20H29BF2N2O4/c22-17-6-3-13(9-18(17)23)12-25-15-4-5-16(25)11-14(10-15)20(24,19(26)27)7-1-2-8-21(28)29/h3,6,9,14-16,28-29H,1-2,4-5,7-8,10-12,24H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642310
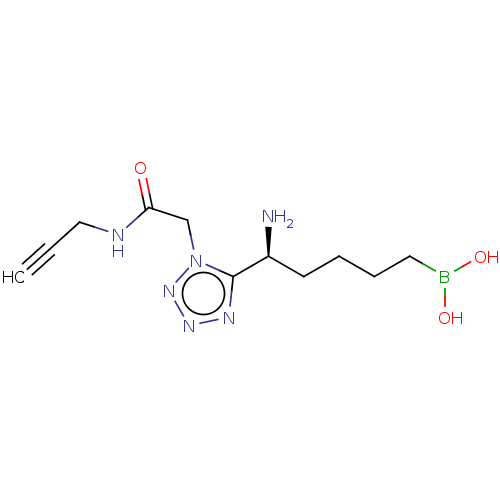
((-)-(S)-(5-amino-5-(1-(2-oxo-2-(prop-2- yn-1-ylami...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439244
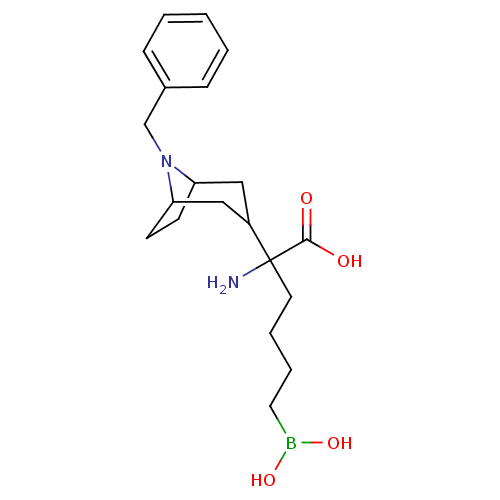
(CHEMBL2418829)Show SMILES NC(CCCCB(O)O)(C1CC2CCC(C1)N2Cc1ccccc1)C(O)=O |TLB:17:16:12.13:9.10.15| Show InChI InChI=1S/C20H31BN2O4/c22-20(19(24)25,10-4-5-11-21(26)27)16-12-17-8-9-18(13-16)23(17)14-15-6-2-1-3-7-15/h1-3,6-7,16-18,26-27H,4-5,8-14,22H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439243
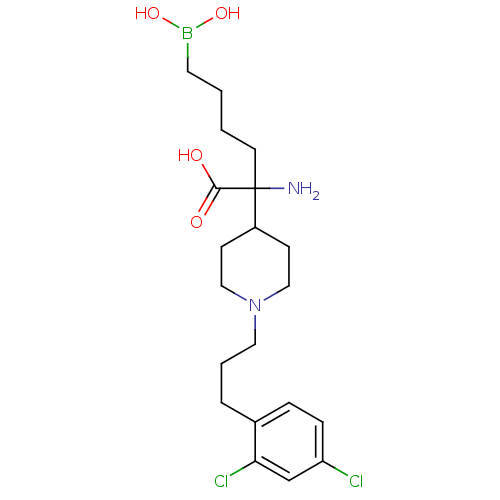
(CHEMBL2418998)Show SMILES NC(CCCCB(O)O)(C1CCN(CCCc2ccc(Cl)cc2Cl)CC1)C(O)=O Show InChI InChI=1S/C20H31BCl2N2O4/c22-17-6-5-15(18(23)14-17)4-3-11-25-12-7-16(8-13-25)20(24,19(26)27)9-1-2-10-21(28)29/h5-6,14,16,28-29H,1-4,7-13,24H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50008099
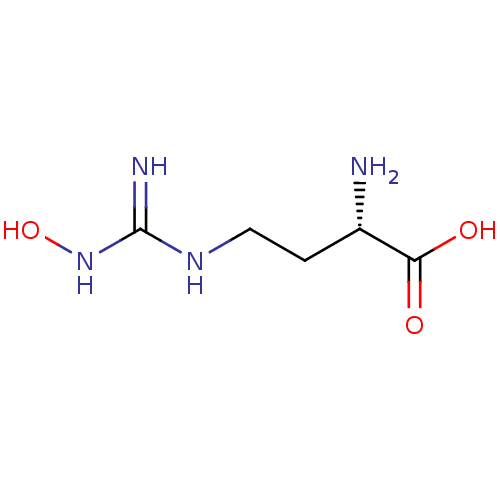
(CHEMBL1234777)Show InChI InChI=1S/C5H12N4O3/c6-3(4(10)11)1-2-8-5(7)9-12/h3,12H,1-2,6H2,(H,10,11)(H3,7,8,9)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM642289
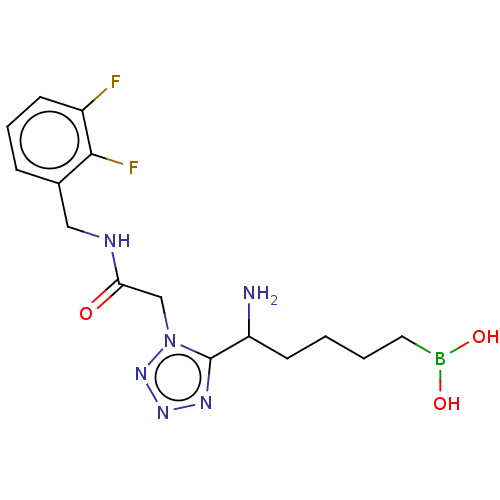
((5-amino-5-(1-(2-((2,3- difluorobenzyl)amino)-2-ox...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50427899
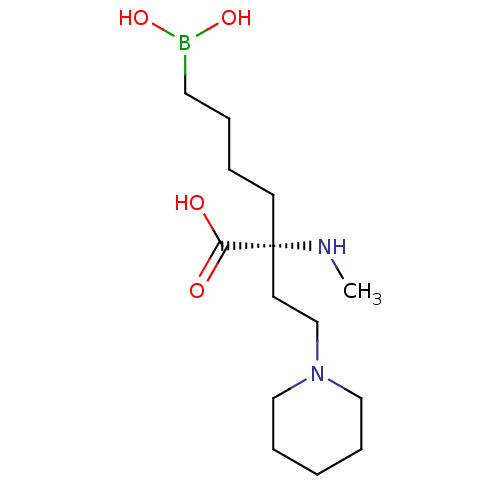
(CHEMBL2326090)Show InChI InChI=1S/C14H29BN2O4/c1-16-14(13(18)19,7-3-4-9-15(20)21)8-12-17-10-5-2-6-11-17/h16,20-21H,2-12H2,1H3,(H,18,19)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant fully active truncated form of arginase 2 overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea ... |
J Med Chem 56: 2568-80 (2013)
Article DOI: 10.1021/jm400014c
BindingDB Entry DOI: 10.7270/Q2BC40WP |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439241

(CHEMBL2418999)Show SMILES NC(CCCCB(O)O)(C1CCN(CCCc2ccc(cc2)C(F)(F)F)CC1)C(O)=O Show InChI InChI=1S/C21H32BF3N2O4/c23-21(24,25)18-7-5-16(6-8-18)4-3-13-27-14-9-17(10-15-27)20(26,19(28)29)11-1-2-12-22(30)31/h5-8,17,30-31H,1-4,9-15,26H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50439242
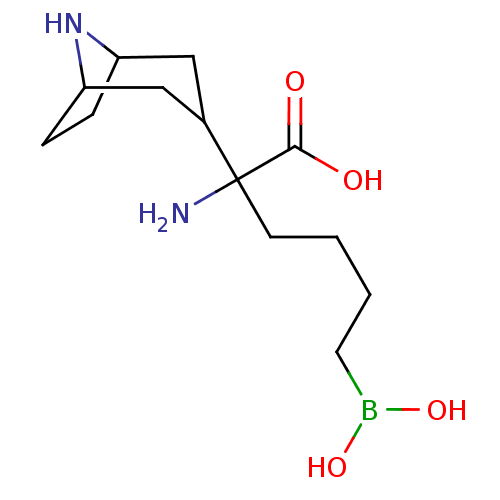
(CHEMBL2418828)Show InChI InChI=1S/C13H25BN2O4/c15-13(12(17)18,5-1-2-6-14(19)20)9-7-10-3-4-11(8-9)16-10/h9-11,16,19-20H,1-8,15H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery
Curated by ChEMBL
| Assay Description
Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay |
Bioorg Med Chem Lett 23: 4837-41 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.092
BindingDB Entry DOI: 10.7270/Q2Z89DT6 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50561049
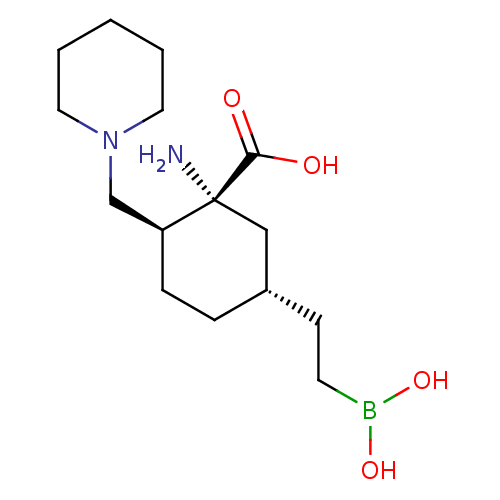
(CHEMBL4793482)Show SMILES N[C@@]1(C[C@H](CCB(O)O)CC[C@H]1CN1CCCCC1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Arg2 |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115658
BindingDB Entry DOI: 10.7270/Q2WS8XZC |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM50595730

(CHEMBL5171566)Show SMILES CC(C)C[C@H](N)C(=O)NC[C@@H]1CC[C@@H](CCB(O)O)C[C@]1(N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00395
BindingDB Entry DOI: 10.7270/Q2VD73H4 |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290430
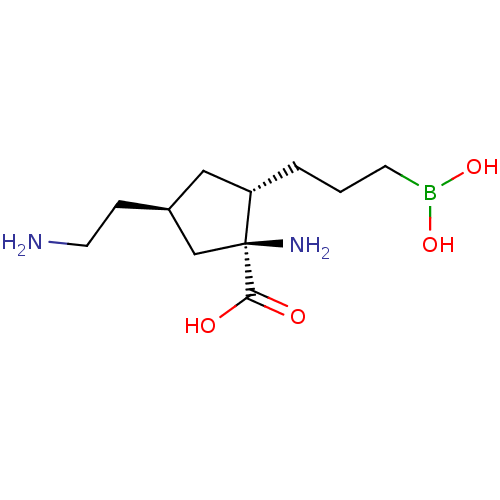
((1S,2S,4R)-1-amino-4-(2-aminoethyl)-2-(3- boronopr...)Show SMILES NCC[C@H]1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O |r| Show InChI InChI=1S/C11H23BN2O4/c13-5-3-8-6-9(2-1-4-12(17)18)11(14,7-8)10(15)16/h8-9,17-18H,1-7,13-14H2,(H,15,16)/t8-,9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290368
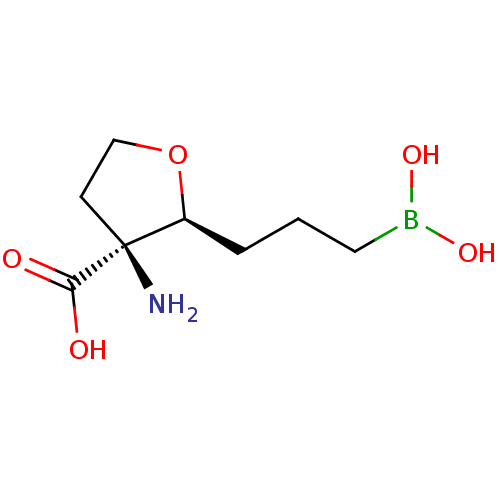
((2S,3S)-3-amino-2-(3-boronopropyl)tetrahydrofuran-...)Show InChI InChI=1S/C8H16BNO5/c10-8(7(11)12)3-5-15-6(8)2-1-4-9(13)14/h6,13-14H,1-5,10H2,(H,11,12)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290378

((3R,4S)-3-amino-1-(2-aminocyclopentyl)-4-(3- boron...)Show SMILES NC1CCCC1N1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O |r| Show InChI InChI=1S/C13H26BN3O4/c15-10-4-1-5-11(10)17-7-9(3-2-6-14(20)21)13(16,8-17)12(18)19/h9-11,20-21H,1-8,15-16H2,(H,18,19)/t9-,10?,11?,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290380
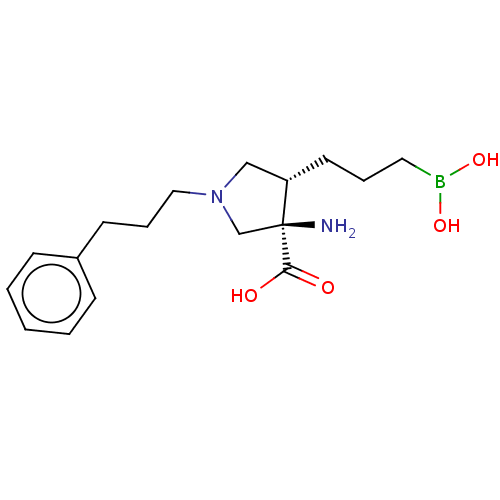
((3R,4S)-3-amino-4-(3-boronopropyl)-1-(3-(4- carbox...)Show SMILES N[C@]1(CN(CCCc2ccccc2)C[C@@H]1CCCB(O)O)C(O)=O |r| Show InChI InChI=1S/C17H27BN2O4/c19-17(16(21)22)13-20(12-15(17)9-4-10-18(23)24)11-5-8-14-6-2-1-3-7-14/h1-3,6-7,15,23-24H,4-5,8-13,19H2,(H,21,22)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290385
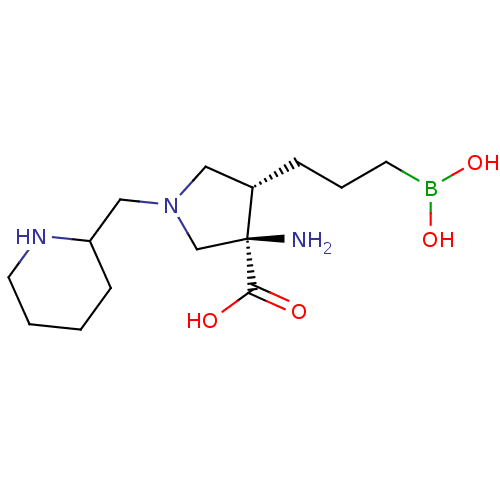
((3R,4S)-3-amino-4-(3-boronopropyl)-1-(piperidin-2-...)Show SMILES N[C@]1(CN(CC2CCCCN2)C[C@@H]1CCCB(O)O)C(O)=O |r| Show InChI InChI=1S/C14H28BN3O4/c16-14(13(19)20)10-18(9-12-5-1-2-7-17-12)8-11(14)4-3-6-15(21)22/h11-12,17,21-22H,1-10,16H2,(H,19,20)/t11-,12?,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290388
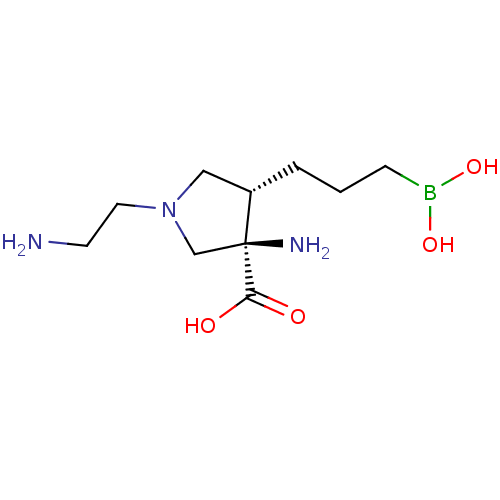
((3R,4S)-3-amino-1-(2-aminoethyl)-4-(3-boronopropyl...)Show SMILES NCCN1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O |r| Show InChI InChI=1S/C10H22BN3O4/c12-4-5-14-6-8(2-1-3-11(17)18)10(13,7-14)9(15)16/h8,17-18H,1-7,12-13H2,(H,15,16)/t8-,10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290391
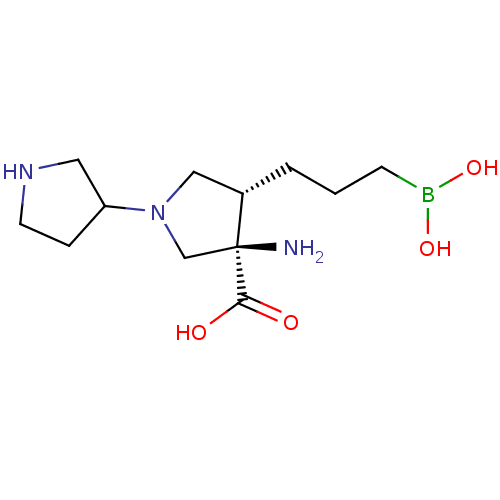
((3R,4S)-3-amino-4-(3-boronopropyl)-1,3'-bipyrrolid...)Show SMILES N[C@]1(CN(C[C@@H]1CCCB(O)O)C1CCNC1)C(O)=O |r| Show InChI InChI=1S/C12H24BN3O4/c14-12(11(17)18)8-16(10-3-5-15-6-10)7-9(12)2-1-4-13(19)20/h9-10,15,19-20H,1-8,14H2,(H,17,18)/t9-,10?,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290402
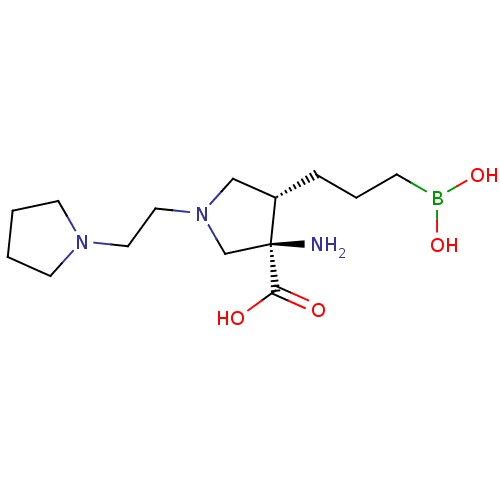
((3R,4S)-3-amino-4-(3-boronopropyl)-1-(2-(pyrrolidi...)Show SMILES N[C@]1(CN(CCN2CCCC2)C[C@@H]1CCCB(O)O)C(O)=O |r| Show InChI InChI=1S/C14H28BN3O4/c16-14(13(19)20)11-18(9-8-17-6-1-2-7-17)10-12(14)4-3-5-15(21)22/h12,21-22H,1-11,16H2,(H,19,20)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290403
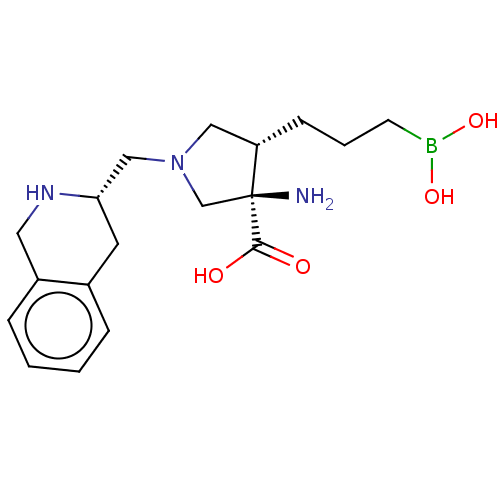
((3R,4S)-3-amino-4-(3-boronopropyl)-1-(((S)-1,2,3,4...)Show SMILES N[C@]1(CN(C[C@@H]2Cc3ccccc3CN2)C[C@@H]1CCCB(O)O)C(O)=O |r| Show InChI InChI=1S/C18H28BN3O4/c20-18(17(23)24)12-22(10-15(18)6-3-7-19(25)26)11-16-8-13-4-1-2-5-14(13)9-21-16/h1-2,4-5,15-16,21,25-26H,3,6-12,20H2,(H,23,24)/t15-,16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290404
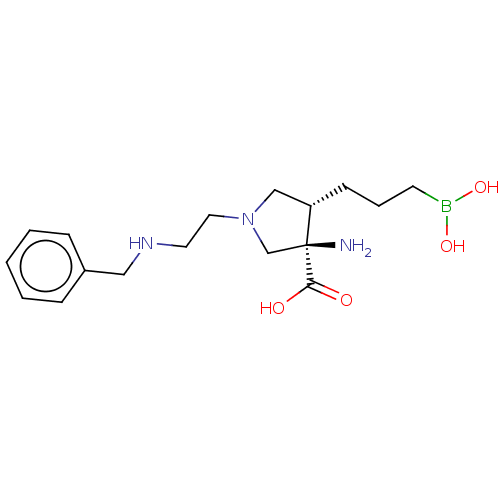
((3R,4S)-3-amino-1-(2-(benzylamino)ethyl)-4-(3- bor...)Show SMILES N[C@]1(CN(CCNCc2ccccc2)C[C@@H]1CCCB(O)O)C(O)=O |r| Show InChI InChI=1S/C17H28BN3O4/c19-17(16(22)23)13-21(12-15(17)7-4-8-18(24)25)10-9-20-11-14-5-2-1-3-6-14/h1-3,5-6,15,20,24-25H,4,7-13,19H2,(H,22,23)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290405
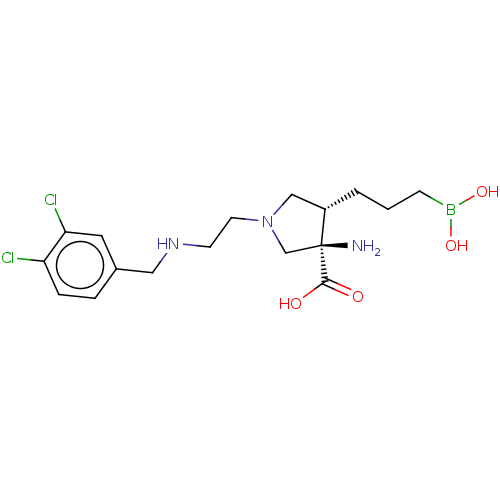
((3R,4S)-3-amino-4-(3-boronopropyl)-1-(2-(3,4- dich...)Show SMILES N[C@]1(CN(CCNCc2ccc(Cl)c(Cl)c2)C[C@@H]1CCCB(O)O)C(O)=O |r| Show InChI InChI=1S/C17H26BCl2N3O4/c19-14-4-3-12(8-15(14)20)9-22-6-7-23-10-13(2-1-5-18(26)27)17(21,11-23)16(24)25/h3-4,8,13,22,26-27H,1-2,5-7,9-11,21H2,(H,24,25)/t13-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290408
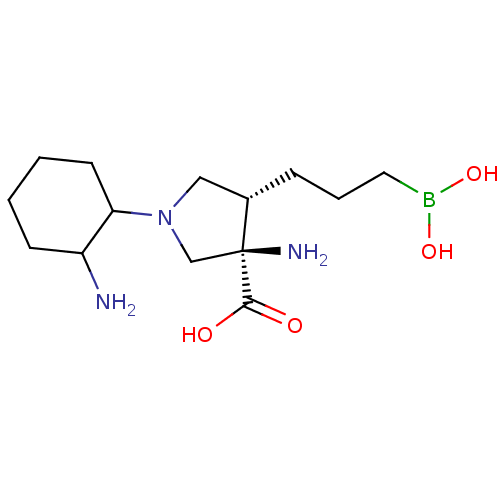
((3R,4S)-3-amino-1-(2-aminocyclohexyl)-4-(3- borono...)Show SMILES NC1CCCCC1N1C[C@H](CCCB(O)O)[C@@](N)(C1)C(O)=O |r| Show InChI InChI=1S/C14H28BN3O4/c16-11-5-1-2-6-12(11)18-8-10(4-3-7-15(21)22)14(17,9-18)13(19)20/h10-12,21-22H,1-9,16-17H2,(H,19,20)/t10-,11?,12?,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |
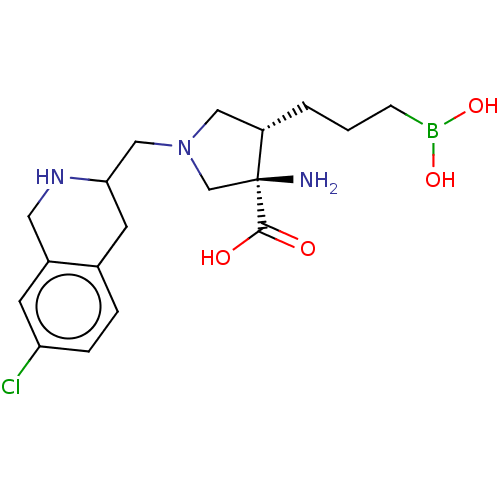
Arginase-2, mitochondrial
(Homo sapiens (Human)) | BDBM290413
((3R,4S)-3-amino-4-(3-boronopropyl)-1-((7-chloro-1,...)Show SMILES N[C@]1(CN(CC2Cc3ccc(Cl)cc3CN2)C[C@@H]1CCCB(O)O)C(O)=O |r| Show InChI InChI=1S/C18H27BClN3O4/c20-15-4-3-12-7-16(22-8-13(12)6-15)10-23-9-14(2-1-5-19(26)27)18(21,11-23)17(24)25/h3-4,6,14,16,22,26-27H,1-2,5,7-11,21H2,(H,24,25)/t14-,16?,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q289193N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data