

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN (Homo sapiens (Human)) | BDBM50388723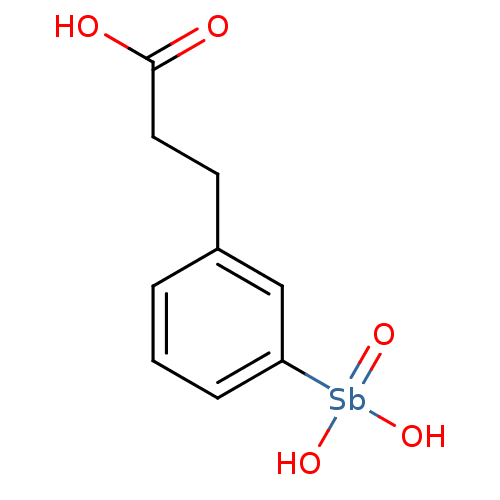 (CHEMBL2057662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human PTEN using 3-O-Methylfluorescein phosphate as substrate incubated for 10 mins prior to substrate addition by fluorometry | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN (Homo sapiens (Human)) | BDBM26613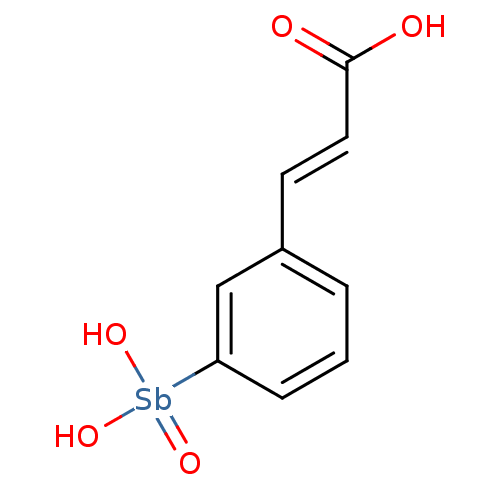 ((2E)-3-{3-[dihydroxy(oxo)--stibanyl]phenyl}prop-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human PTEN using 3-O-Methylfluorescein phosphate as substrate incubated for 10 mins prior to substrate addition by fluorometry | Bioorg Med Chem 20: 4371-6 (2012) Article DOI: 10.1016/j.bmc.2012.05.040 BindingDB Entry DOI: 10.7270/Q2RB75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN (Homo sapiens (Human)) | BDBM50071058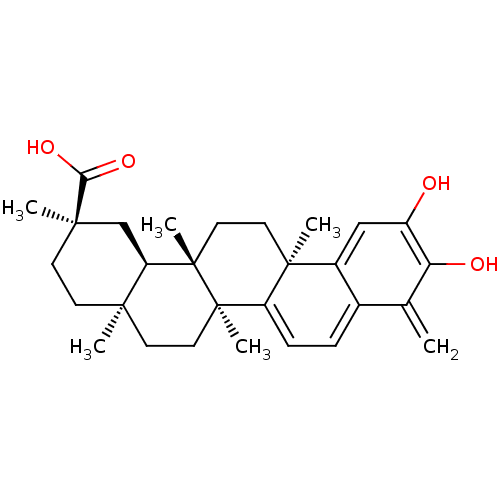 ((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz Zentrum M�nchen Curated by ChEMBL | Assay Description Inhibition of human PTEN (1 to 403 residues) expressed in Escherichia coli strain BL21(DE3) using DiFMUP as substrate preincubated for 10 mins follow... | J Med Chem 61: 11144-11157 (2018) Article DOI: 10.1021/acs.jmedchem.8b01224 BindingDB Entry DOI: 10.7270/Q2G44TN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||