

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50434850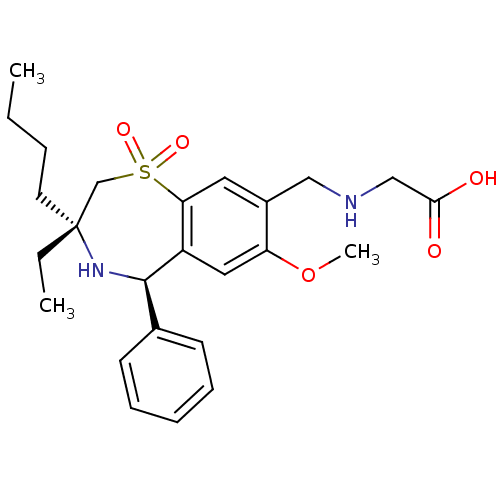 (CHEMBL2387397) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50434850 (CHEMBL2387397) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50434849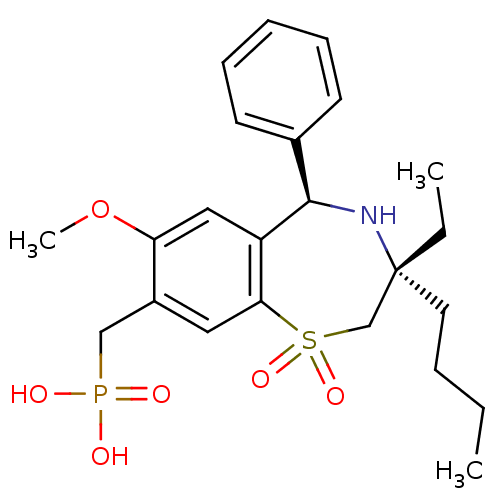 (CHEMBL2385105 | US9040518, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50434846 (CHEMBL2387399 | US9040518, 35) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50434849 (CHEMBL2385105 | US9040518, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM47370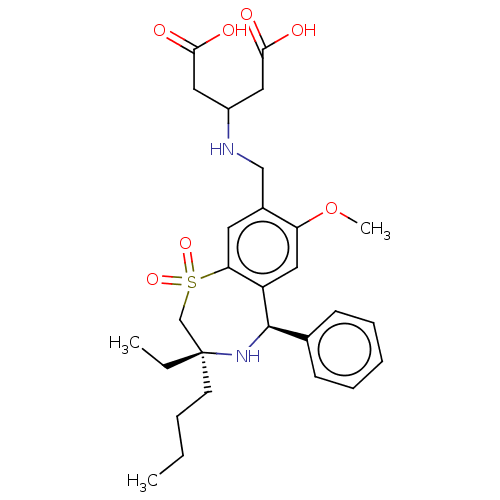 (BDBM50434858 | US9040518, 26) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM47370 (BDBM50434858 | US9040518, 26) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50434846 (CHEMBL2387399 | US9040518, 35) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50134411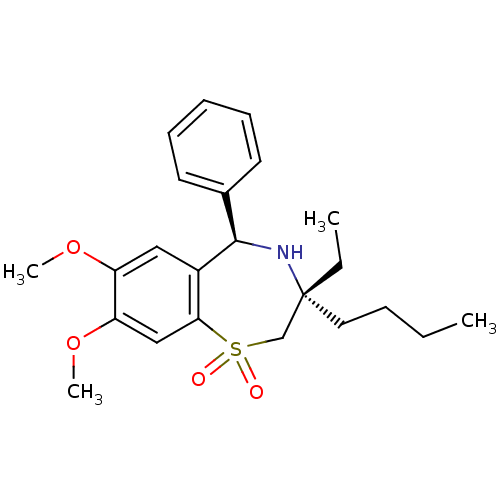 ((7R,9R)-7-Butyl-7-ethyl-2,3-dimethoxy-9-phenyl-6,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50134411 ((7R,9R)-7-Butyl-7-ethyl-2,3-dimethoxy-9-phenyl-6,7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50459098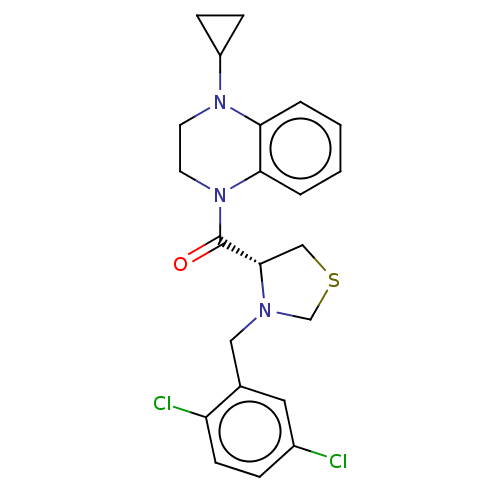 (CHEMBL4205214) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ardelyx, Inc. Curated by ChEMBL | Assay Description Inhibition of mouse IBAT expressed in COS cells assessed as reduction in [3H]taurocholic acid uptake | J Med Chem 61: 7589-7613 (2018) Article DOI: 10.1021/acs.jmedchem.8b00308 BindingDB Entry DOI: 10.7270/Q2B56NCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50459099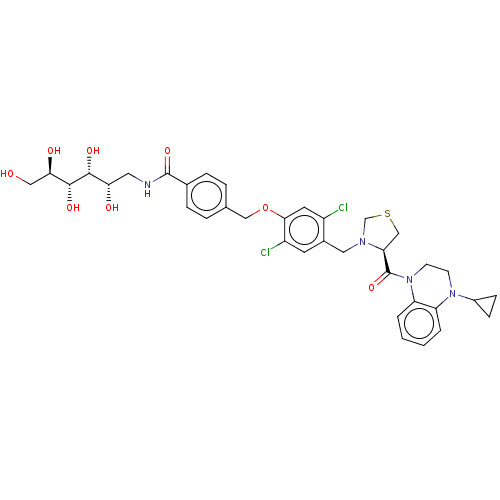 (CHEMBL4202981) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ardelyx, Inc. Curated by ChEMBL | Assay Description Inhibition of mouse IBAT expressed in COS cells assessed as reduction in [3H]taurocholic acid uptake | J Med Chem 61: 7589-7613 (2018) Article DOI: 10.1021/acs.jmedchem.8b00308 BindingDB Entry DOI: 10.7270/Q2B56NCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50459096 (CHEMBL4217797) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ardelyx, Inc. Curated by ChEMBL | Assay Description Inhibition of mouse IBAT expressed in COS cells assessed as reduction in [3H]taurocholic acid uptake | J Med Chem 61: 7589-7613 (2018) Article DOI: 10.1021/acs.jmedchem.8b00308 BindingDB Entry DOI: 10.7270/Q2B56NCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||