

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50581255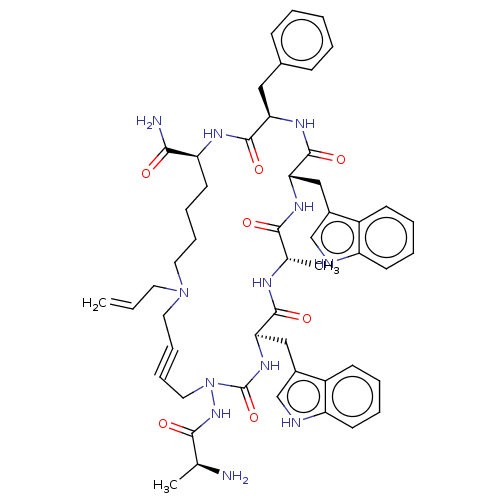 (CHEMBL4531645) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Tyr-Bpa-Ala-hexarelin from rat CD36 receptor in heart tissue membrane incubated for 60 mins by competitive receptor binding as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00642 BindingDB Entry DOI: 10.7270/Q21C21RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50581250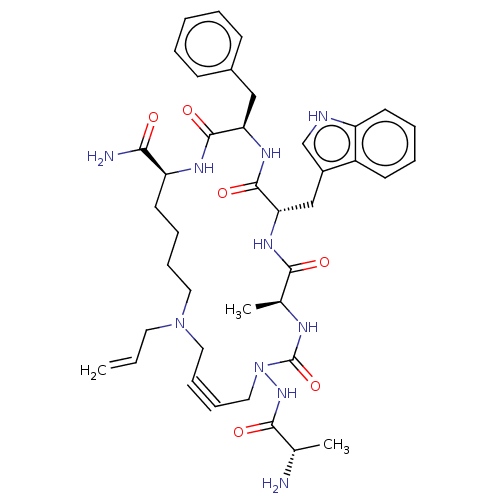 (CHEMBL4455562) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Tyr-Bpa-Ala-hexarelin from rat CD36 receptor in heart tissue membrane incubated for 60 mins by competitive receptor binding as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00642 BindingDB Entry DOI: 10.7270/Q21C21RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50581260 (CHEMBL4528721) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Tyr-Bpa-Ala-hexarelin from rat CD36 receptor in heart tissue membrane incubated for 60 mins by competitive receptor binding as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00642 BindingDB Entry DOI: 10.7270/Q21C21RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50581254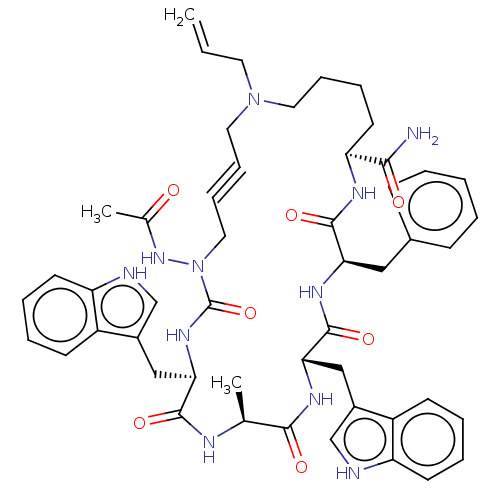 (CHEMBL4471086) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Tyr-Bpa-Ala-hexarelin from rat CD36 receptor in heart tissue membrane incubated for 60 mins by competitive receptor binding as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00642 BindingDB Entry DOI: 10.7270/Q21C21RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395009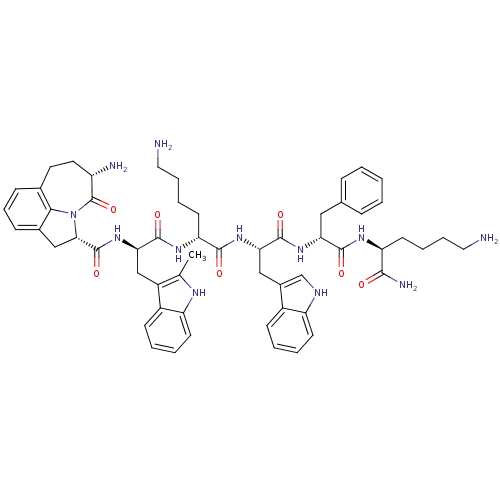 (CHEMBL2163470) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395001 (CHEMBL2163478 | US9708370, DBG-253-1) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395001 (CHEMBL2163478 | US9708370, DBG-253-1) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50370294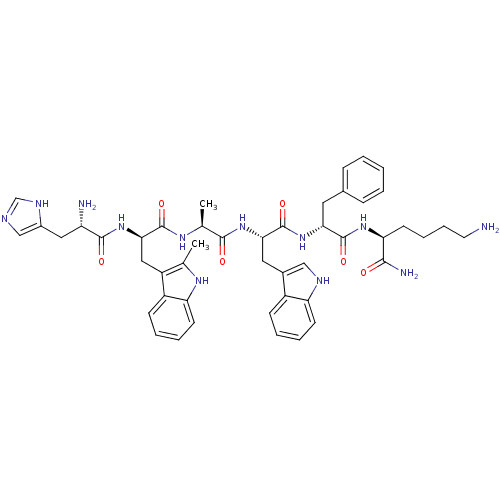 (Examorelin | HEXARELIN | US9708370, Hexareline) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of photoactivatable [125I]-Tyr-Bpa-Ala-hexarelin binding to CD36 in Sprague-Dawley rat heart membranes after 60 mins by SDS-PAGE analysis | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50242203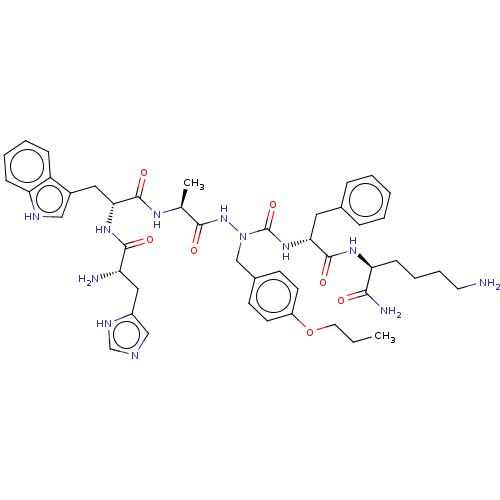 (CHEMBL4078128) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of photoactivatable [125I]-Tyr-Bpa-Ala-hexarelin binding to CD36 in Sprague-Dawley rat heart membranes after 60 mins by SDS-PAGE analysis | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395007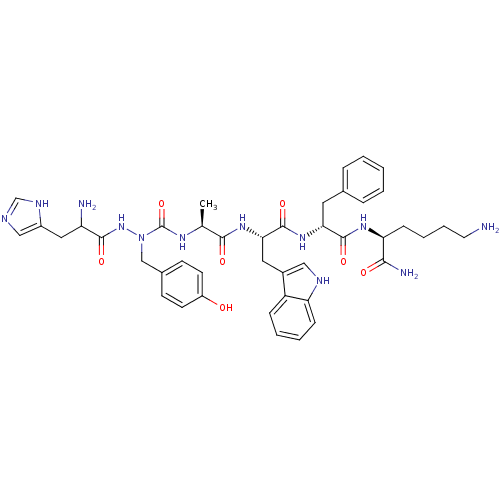 (CHEMBL2163472) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM263222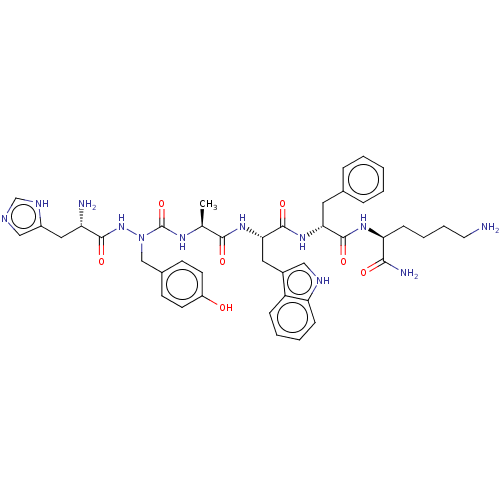 (US9708370, DBG-175p) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50049479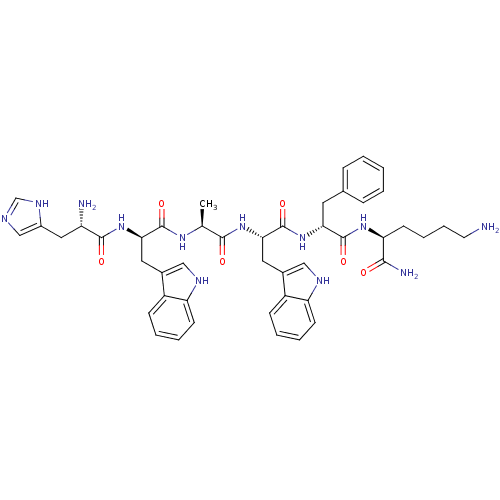 ((S)-6-Amino-2-{(R)-2-[(S)-2-{(S)-2-[(R)-2-[(S)-2-a...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50581259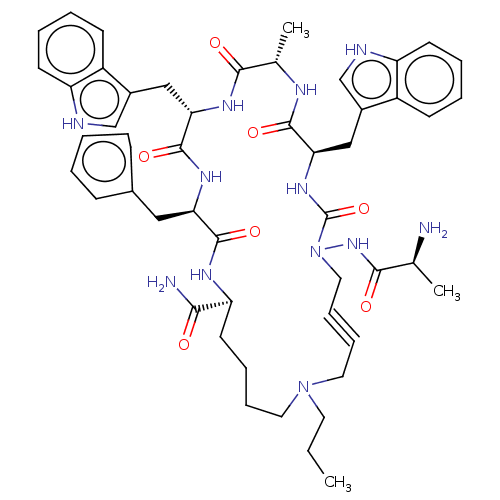 (CHEMBL5080816) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Tyr-Bpa-Ala-hexarelin from rat CD36 receptor in heart tissue membrane incubated for 60 mins by competitive receptor binding as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00642 BindingDB Entry DOI: 10.7270/Q21C21RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50394994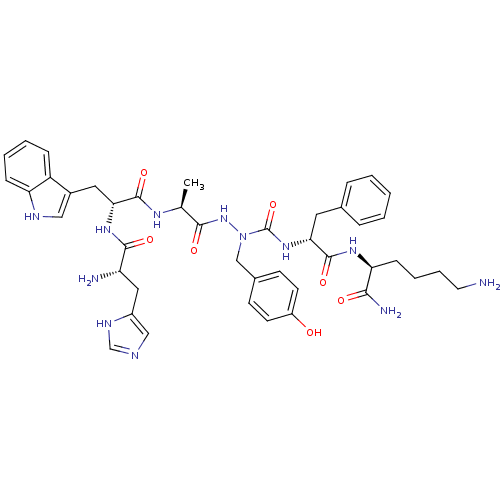 (CHEMBL2163484 | US9708370, DBG-178p) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of photoactivatable [125I]-Tyr-Bpa-Ala-hexarelin binding to CD36 in Sprague-Dawley rat heart membranes after 60 mins by SDS-PAGE analysis | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50370294 (Examorelin | HEXARELIN | US9708370, Hexareline) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50581261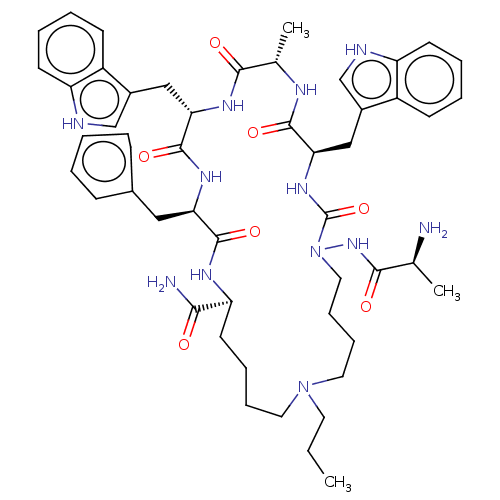 (CHEMBL5092625) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Tyr-Bpa-Ala-hexarelin from rat CD36 receptor in heart tissue membrane incubated for 60 mins by competitive receptor binding as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00642 BindingDB Entry DOI: 10.7270/Q21C21RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395002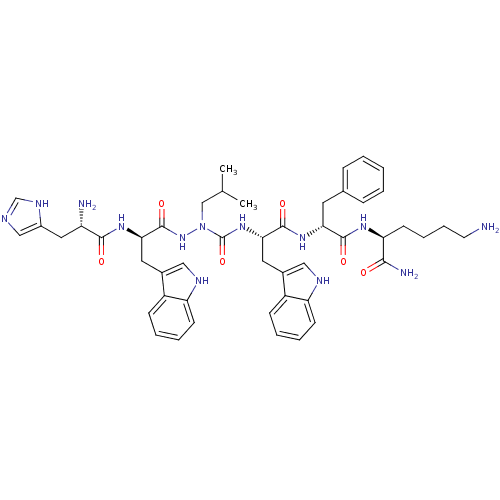 (CHEMBL2163477 | US9708370, DBG-201-A) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395002 (CHEMBL2163477 | US9708370, DBG-201-A) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM263256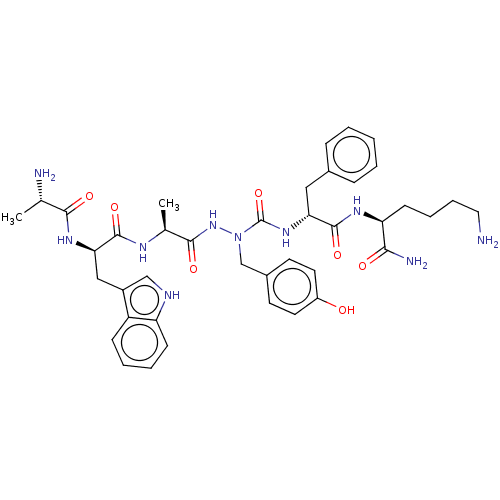 (US9708370, 67 ZS555-F40 | US9708370, ZS555-F40) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50370294 (Examorelin | HEXARELIN | US9708370, Hexareline) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50581258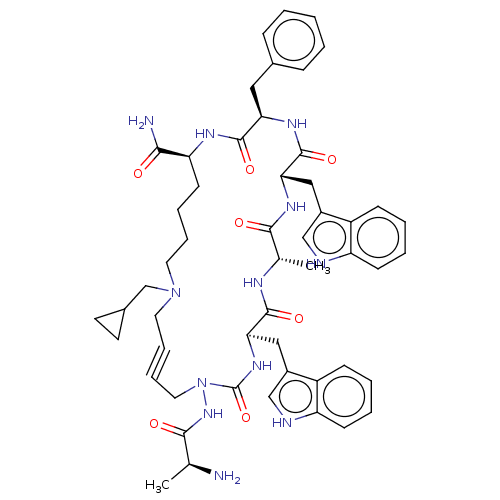 (CHEMBL5094226) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Tyr-Bpa-Ala-hexarelin from rat CD36 receptor in heart tissue membrane incubated for 60 mins by competitive receptor binding as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00642 BindingDB Entry DOI: 10.7270/Q21C21RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50370294 (Examorelin | HEXARELIN | US9708370, Hexareline) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.33E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50242204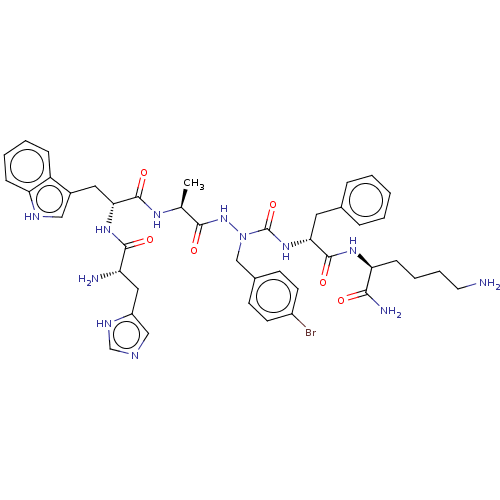 (CHEMBL4086017) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 10% fetal calf serum(FCS) | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395019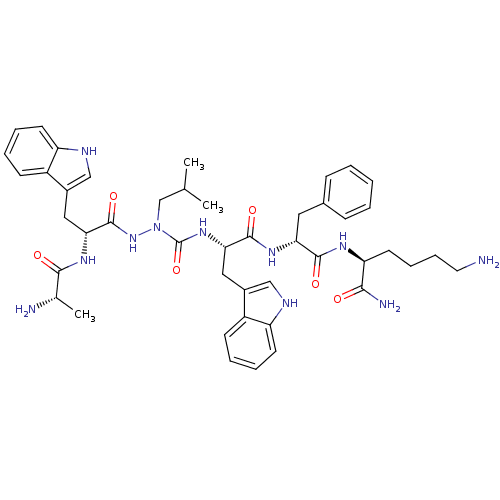 (CHEMBL2163449 | US9708370, CP-2B(v)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50581256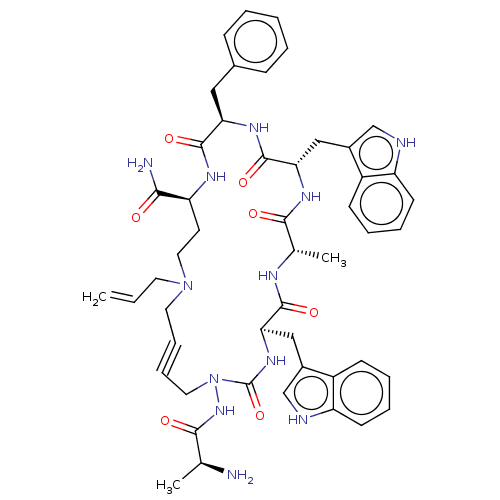 (CHEMBL5087491) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Tyr-Bpa-Ala-hexarelin from rat CD36 receptor in heart tissue membrane incubated for 60 mins by competitive receptor binding as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00642 BindingDB Entry DOI: 10.7270/Q21C21RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395016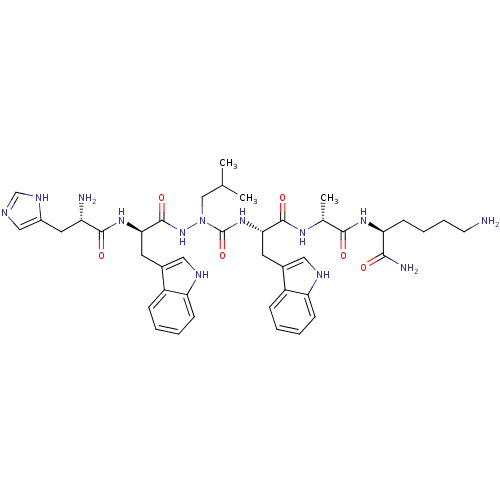 (CHEMBL2163452 | US9708370, CP-2B(ii)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.94E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50242206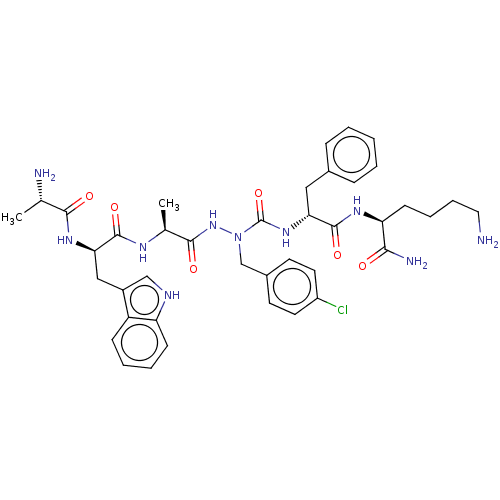 (CHEMBL4080592) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of photoactivatable [125I]-Tyr-Bpa-Ala-hexarelin binding to CD36 in Sprague-Dawley rat heart membranes after 60 mins by SDS-PAGE analysis | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50394993 (CHEMBL2163485 | US9708370, CP-1A(iv)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.02E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50242210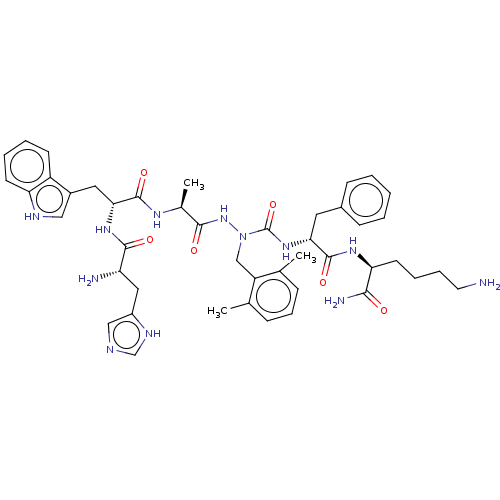 (CHEMBL4104823) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of photoactivatable [125I]-Tyr-Bpa-Ala-hexarelin binding to CD36 in Sprague-Dawley rat heart membranes after 60 mins by SDS-PAGE analysis | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50242216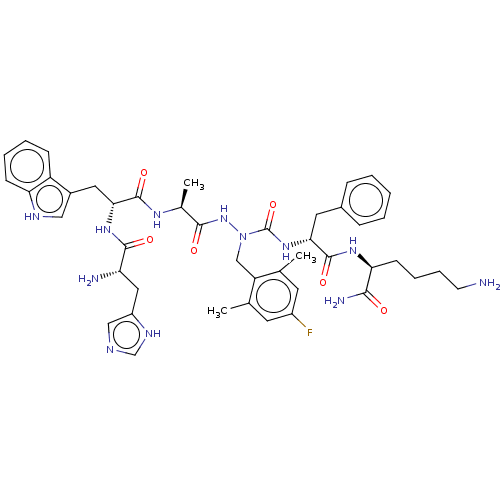 (CHEMBL4088461) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 10% fetal calf serum(FCS) | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395024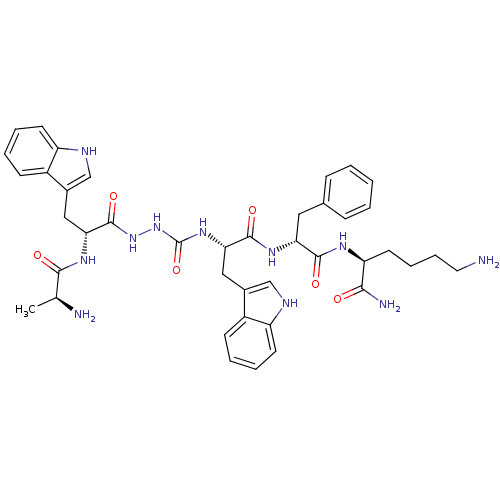 (CHEMBL2163444 | US9708370, CP-2A(v)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50242211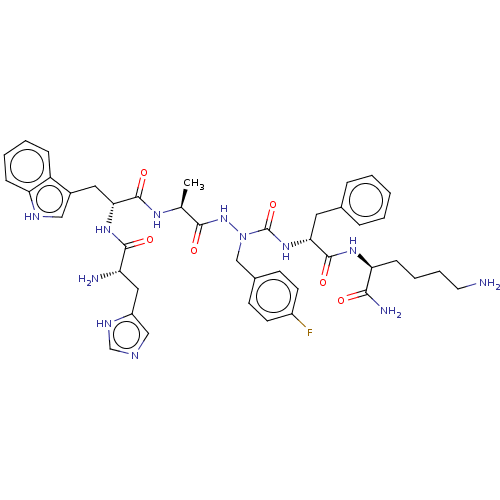 (CHEMBL4067202) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of photoactivatable [125I]-Tyr-Bpa-Ala-hexarelin binding to CD36 in Sprague-Dawley rat heart membranes after 60 mins by SDS-PAGE analysis | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395019 (CHEMBL2163449 | US9708370, CP-2B(v)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50242212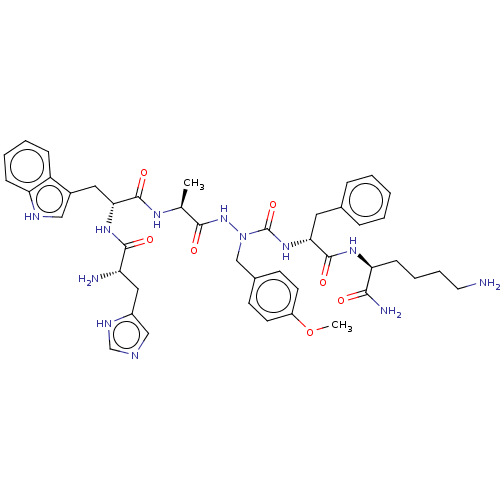 (CHEMBL4085058) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-2 protease in the absence of DMSO | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395014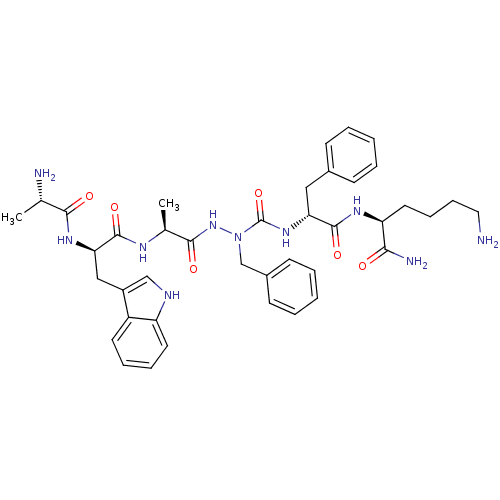 (CHEMBL2163454 | US9708370, 60 CP-3(iv)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395015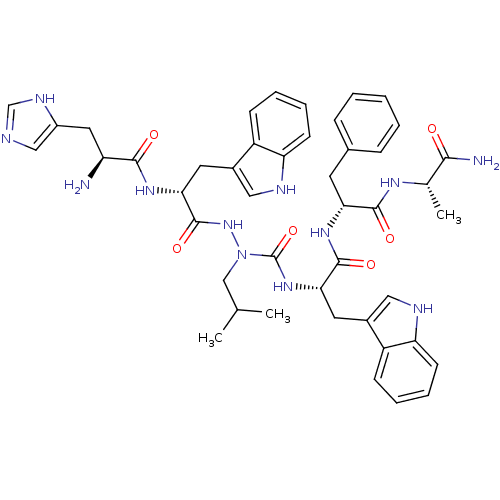 (CHEMBL2163453 | US9708370, CP-2B(i)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50394983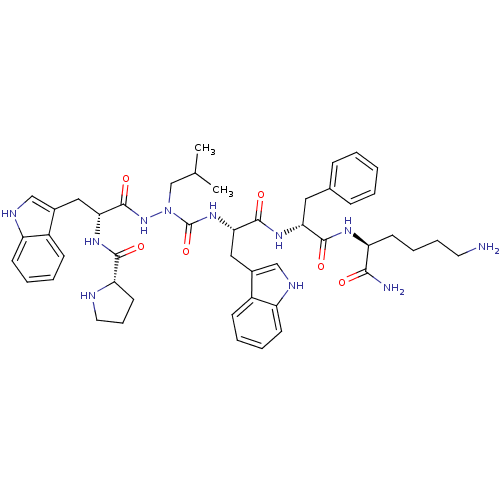 (CHEMBL2163466) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50242213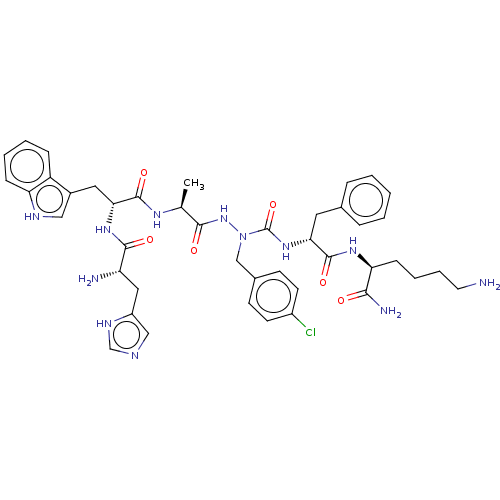 (CHEMBL4063430) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease in 10% fetal calf serum(FCS) | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395024 (CHEMBL2163444 | US9708370, CP-2A(v)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.76E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50394982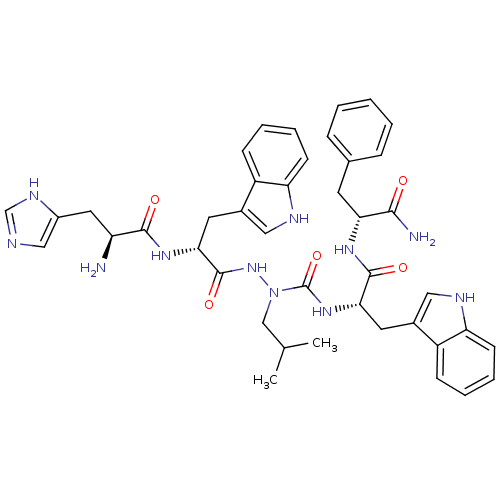 (CHEMBL2163467) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395015 (CHEMBL2163453 | US9708370, CP-2B(i)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.02E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50581262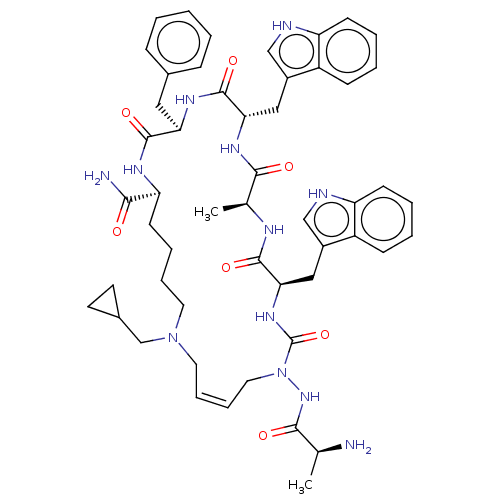 (CHEMBL5086116) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Tyr-Bpa-Ala-hexarelin from rat CD36 receptor in heart tissue membrane incubated for 60 mins by competitive receptor binding as... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00642 BindingDB Entry DOI: 10.7270/Q21C21RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM263256 (US9708370, 67 ZS555-F40 | US9708370, ZS555-F40) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Inhibition of photoactivatable [125I]-Tyr-Bpa-Ala-hexarelin binding to CD36 in Sprague-Dawley rat heart membranes after 60 mins by SDS-PAGE analysis | J Med Chem 60: 9263-9274 (2017) Article DOI: 10.1021/acs.jmedchem.7b01209 BindingDB Entry DOI: 10.7270/Q2GF0WWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395016 (CHEMBL2163452 | US9708370, CP-2B(ii)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395003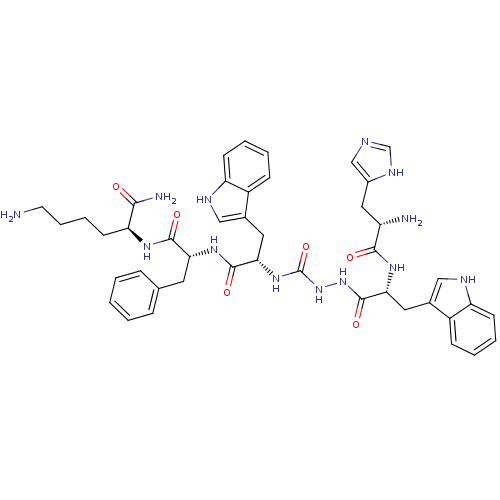 (CHEMBL2163476 | US9708370, DBG-188p) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395003 (CHEMBL2163476 | US9708370, DBG-188p) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.61E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395017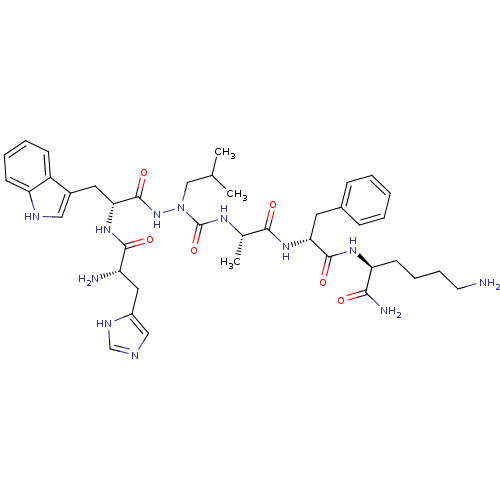 (CHEMBL2163451 | US9708370, CP-2B(iii)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.66E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50394989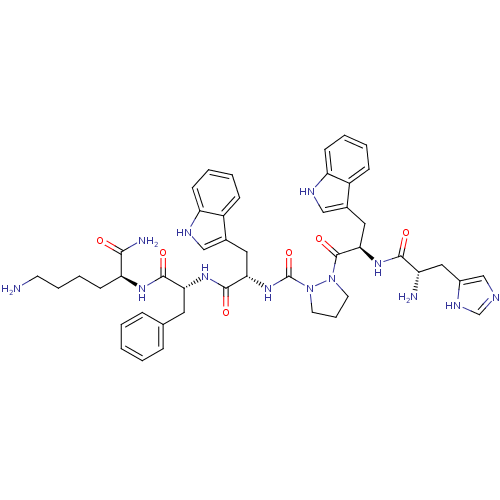 (CHEMBL2163460 | US9708370, 62 CP-AP-4) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50394991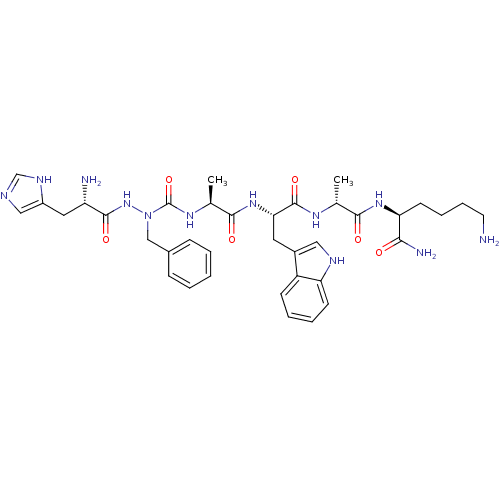 (CHEMBL2163438 | US9708370, 40 CP-1A(ii)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50394992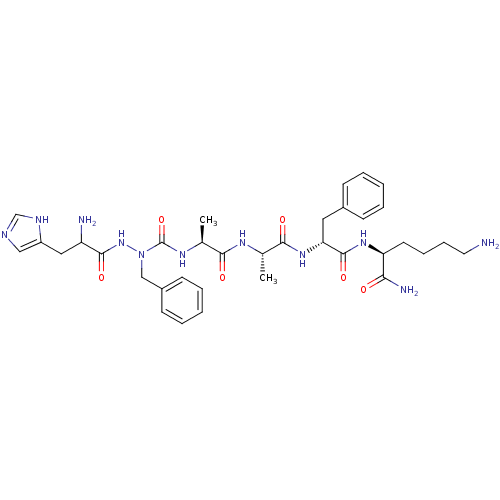 (CHEMBL2163437) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of photoactivatable [125I]-Tyr-Bpa-Ala-Hexarelin from CD36 in Sprague-Dawley rat cardiac membranes incubated for 60 mins by densitometry | J Med Chem 55: 6502-11 (2012) Article DOI: 10.1021/jm300557t BindingDB Entry DOI: 10.7270/Q22J6D00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 117 total ) | Next | Last >> |