

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249571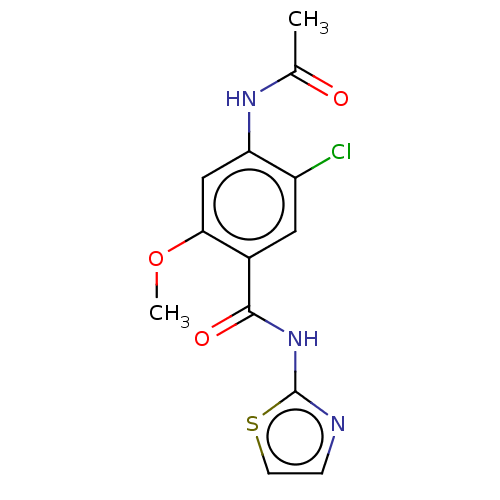 (CHEMBL4101927 | US10729695, Compound CB-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249571 (CHEMBL4101927 | US10729695, Compound CB-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Inhibition of CML binding to RAGE (unknown origin) by ELISA | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM95323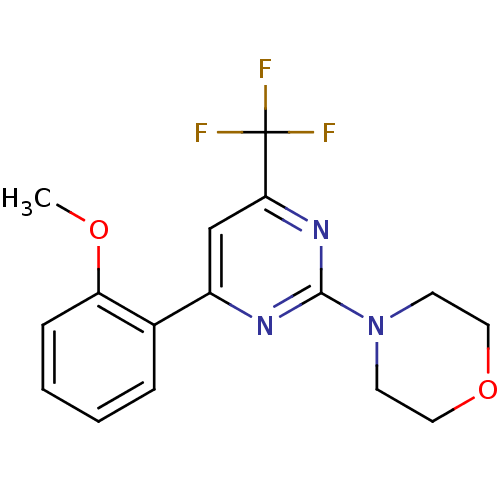 (4-[4-(2-methoxyphenyl)-6-(trifluoromethyl)-2-pyrim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249579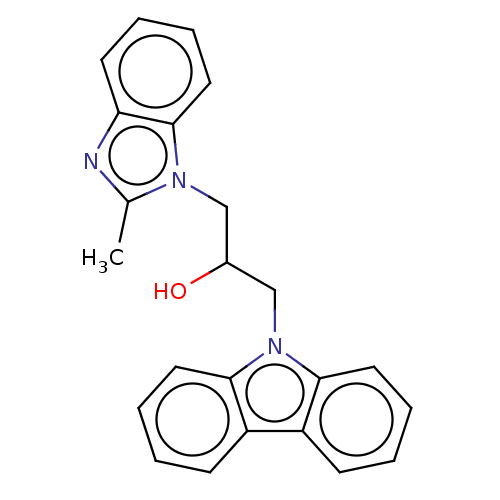 (CHEMBL1477694 | US10729695, Compound CB-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Inhibition of CML binding to RAGE (unknown origin) by ELISA | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249574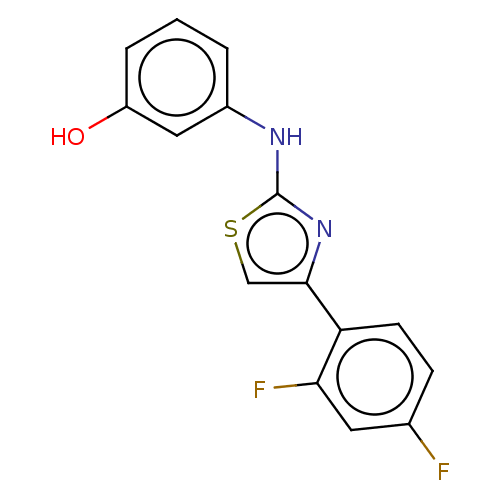 (CHEMBL4077165 | US10729695, Compound CB-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Inhibition of CML binding to RAGE (unknown origin) by ELISA | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249574 (CHEMBL4077165 | US10729695, Compound CB-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01571 BindingDB Entry DOI: 10.7270/Q25H7MBX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249574 (CHEMBL4077165 | US10729695, Compound CB-5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM32273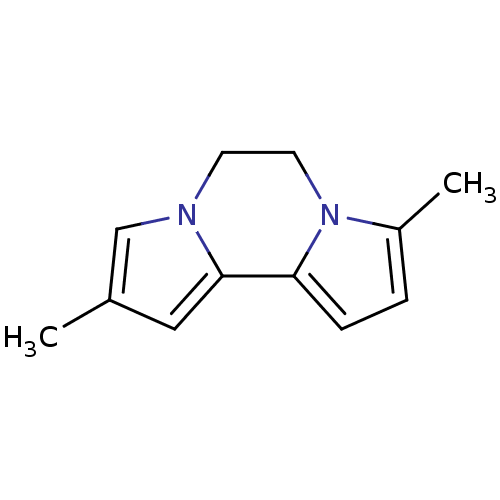 (2,8-dimethyl-5,6-dihydrodipyrrolo[1,2-b:1',2'-e]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453281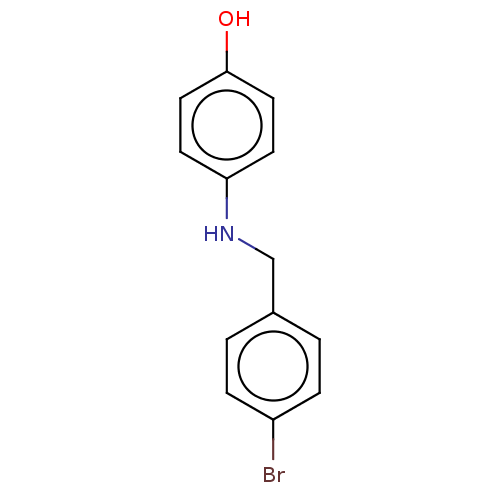 (US10729695, Compound CB-14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249573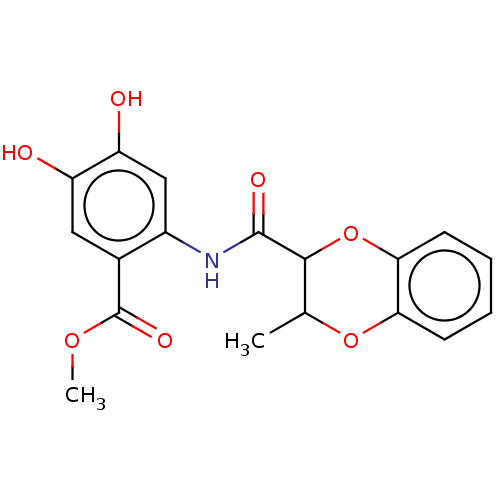 (CHEMBL4063929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Inhibition of CML binding to RAGE (unknown origin) by ELISA | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453279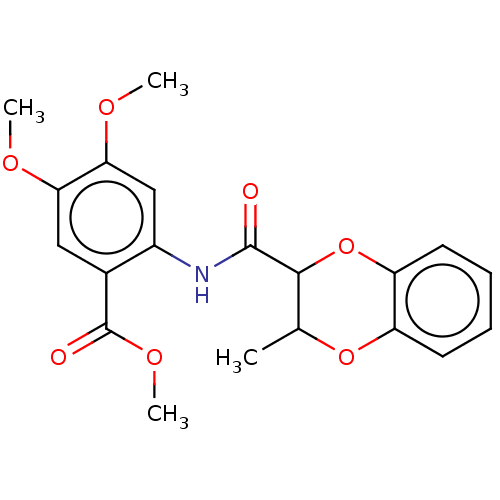 (US10729695, Compound CB-8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249577 (CHEMBL4072531 | US10729695, Compound CB-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249577 (CHEMBL4072531 | US10729695, Compound CB-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Inhibition of CML binding to RAGE (unknown origin) by ELISA | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249575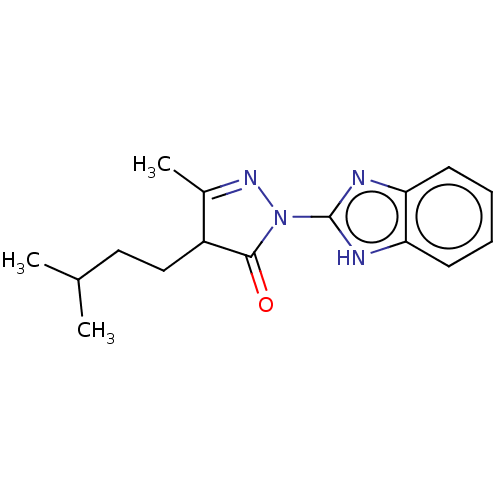 (CHEMBL4100986 | US10729695, Compound CB-10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Inhibition of CML binding to RAGE (unknown origin) by ELISA | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249575 (CHEMBL4100986 | US10729695, Compound CB-10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249576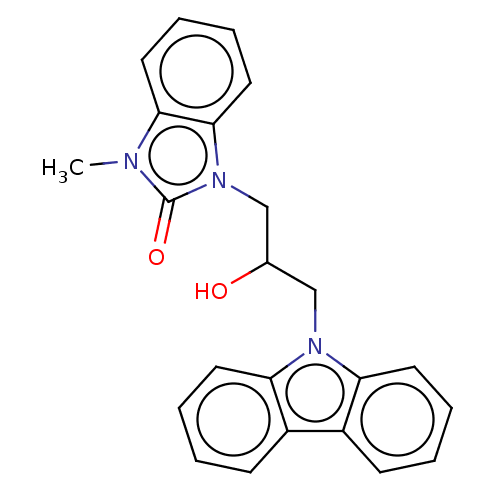 (CHEMBL4075539 | US10729695, Compound CD-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249572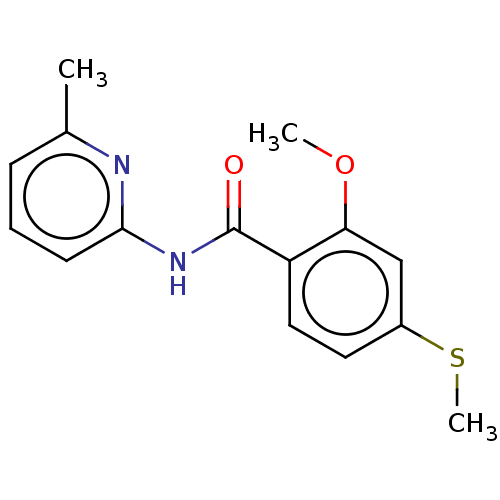 (CHEMBL1463447 | US10729695, Compound CB-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | US Patent | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249576 (CHEMBL4075539 | US10729695, Compound CD-6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Inhibition of CML binding to RAGE (unknown origin) by ELISA | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249572 (CHEMBL1463447 | US10729695, Compound CB-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Inhibition of CML binding to RAGE (unknown origin) by ELISA | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453285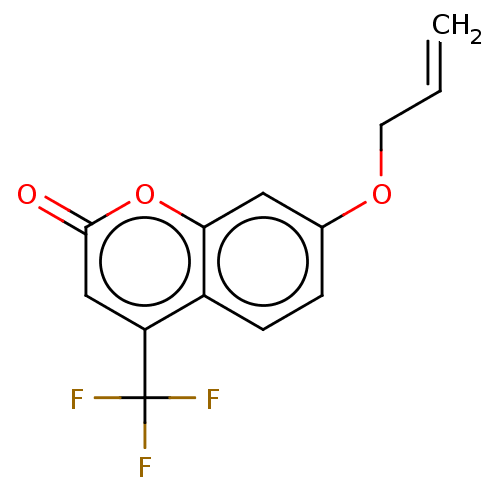 (US10729695, Compound CB-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM582522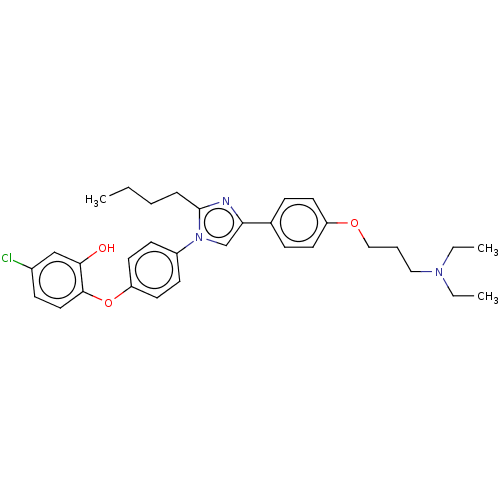 (US11524942, Compound M5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding affinity of COMPOUND I and compounds of the invention to human sRAGE (the soluble extracellular domain of the human receptor for advanced... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM582526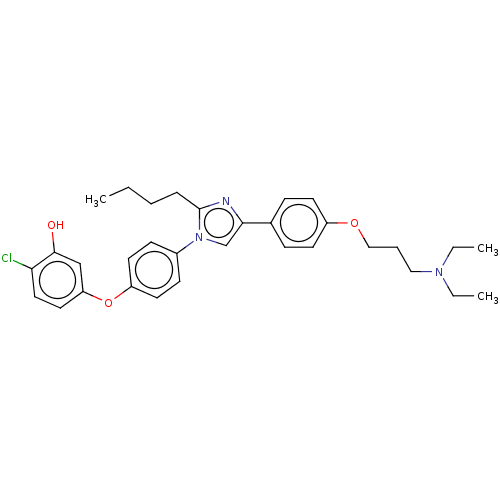 (US11524942, Compound M6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding affinity of COMPOUND I and compounds of the invention to human sRAGE (the soluble extracellular domain of the human receptor for advanced... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249580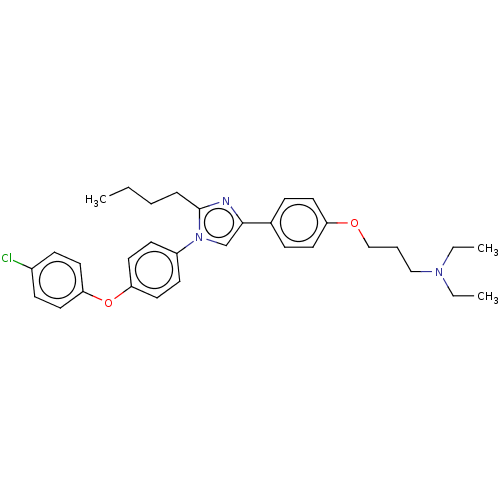 (Azeliragon | PF-04494700 | TTP448 | US11524942, Co...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding affinity of COMPOUND I and compounds of the invention to human sRAGE (the soluble extracellular domain of the human receptor for advanced... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249580 (Azeliragon | PF-04494700 | TTP448 | US11524942, Co...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114347 BindingDB Entry DOI: 10.7270/Q2PG1WTJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249570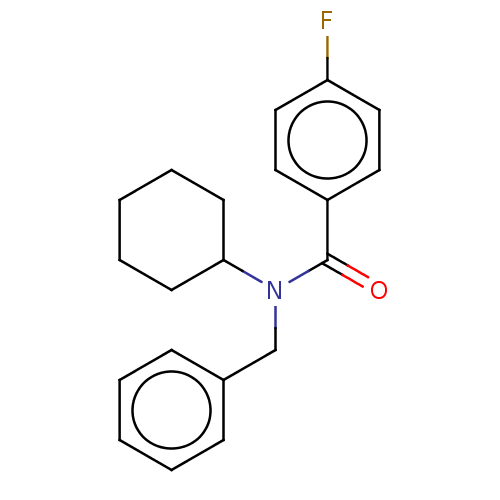 (CHEMBL4066660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01571 BindingDB Entry DOI: 10.7270/Q25H7MBX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249570 (CHEMBL4066660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Binding affinity to human RAGE domain V by autoradiography | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249579 (CHEMBL1477694 | US10729695, Compound CB-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM582520 (US11524942, Compound M2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 22.3 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding affinity of COMPOUND I and compounds of the invention to human sRAGE (the soluble extracellular domain of the human receptor for advanced... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249566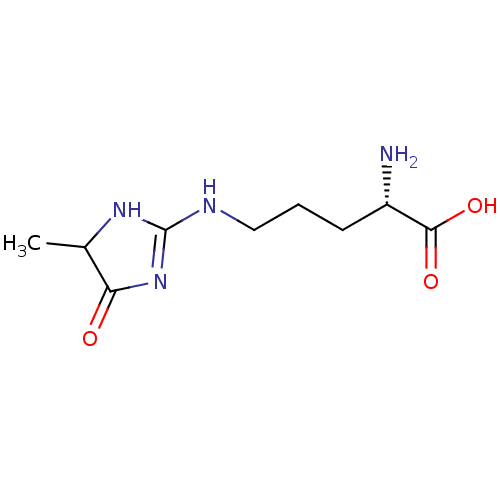 (CHEMBL4085254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Binding affinity to human C-terminal His-tagged RAGE domain V (24 to 125 residues) expressed in Escherichia coli BL21(DE3) by tryptophan fluorescence... | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249582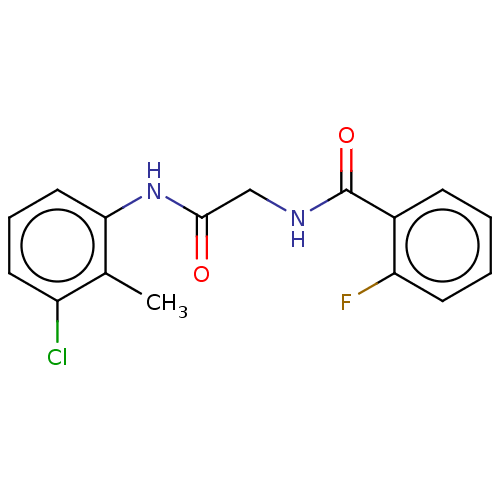 (CHEMBL1547178 | US10729695, Compound CB-13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Inhibition of CML binding to RAGE (unknown origin) by ELISA | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM26115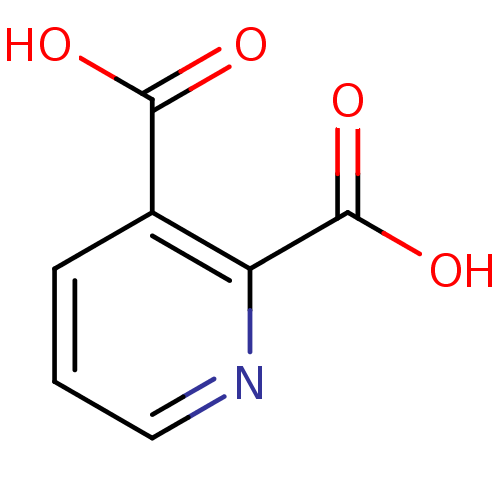 (CHEMBL286204 | Quinolinate | Quinolinic acid | pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Binding affinity to human His-tagged RAGE domain VC1 expressed in Escherichia coli by fluorescence titration method | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453292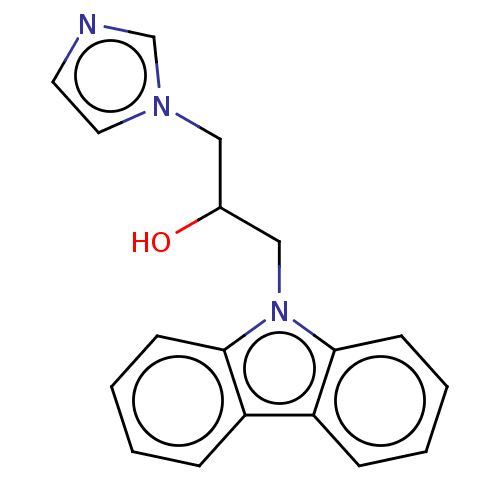 (US10729695, Compound CD-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453304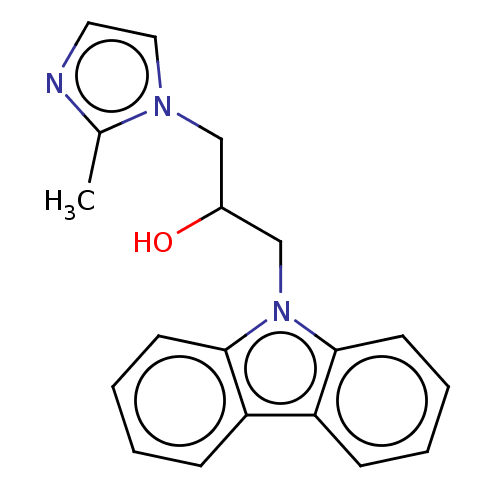 (US10729695, Compound CD-29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM582527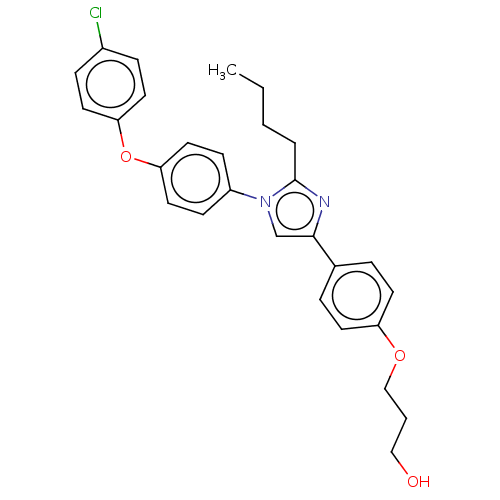 (US11524942, Compound M7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 99.8 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding affinity of COMPOUND I and compounds of the invention to human sRAGE (the soluble extracellular domain of the human receptor for advanced... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249582 (CHEMBL1547178 | US10729695, Compound CB-13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM582518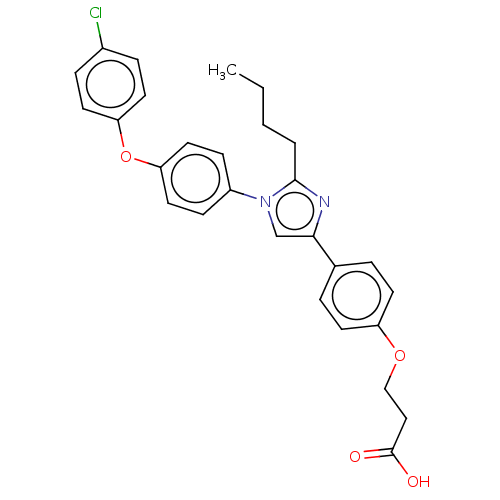 (US11524942, Compound M1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding affinity of COMPOUND I and compounds of the invention to human sRAGE (the soluble extracellular domain of the human receptor for advanced... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM582521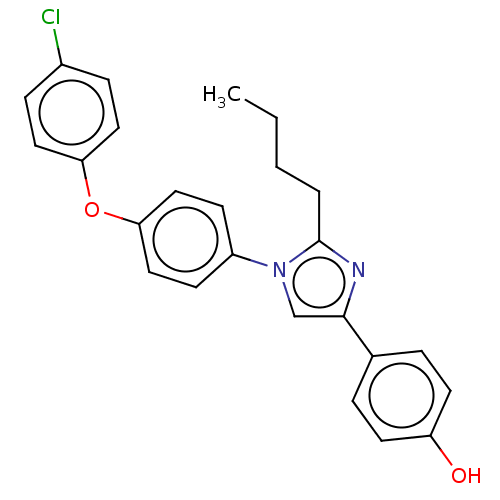 (US11524942, Compound M3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The binding affinity of COMPOUND I and compounds of the invention to human sRAGE (the soluble extracellular domain of the human receptor for advanced... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM50249580 (Azeliragon | PF-04494700 | TTP448 | US11524942, Co...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a |
King's College London Curated by ChEMBL | Assay Description Inhibition of amyloid beta (1 to 42) binding to RAGE domain V (unknown origin) by fluorescence polarization assay | J Med Chem 60: 7213-7232 (2017) Article DOI: 10.1021/acs.jmedchem.7b00058 BindingDB Entry DOI: 10.7270/Q2KD21BP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453288 (US10729695, Compound CB-9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453303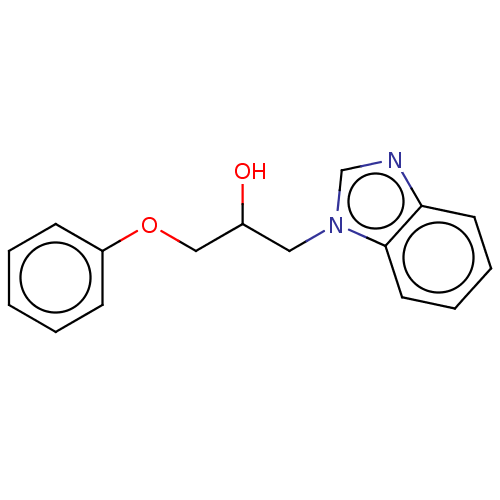 (US10729695, Compound CD-28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453302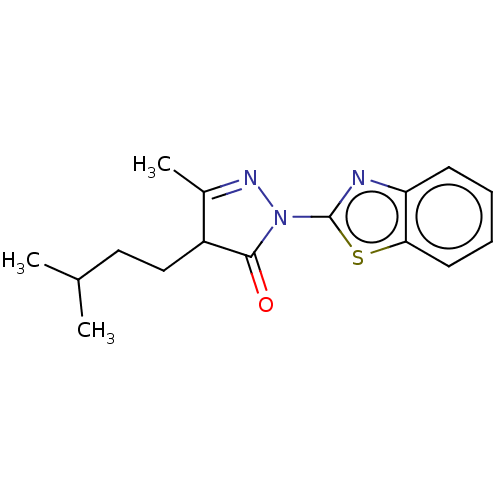 (US10729695, Compound CD-20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453301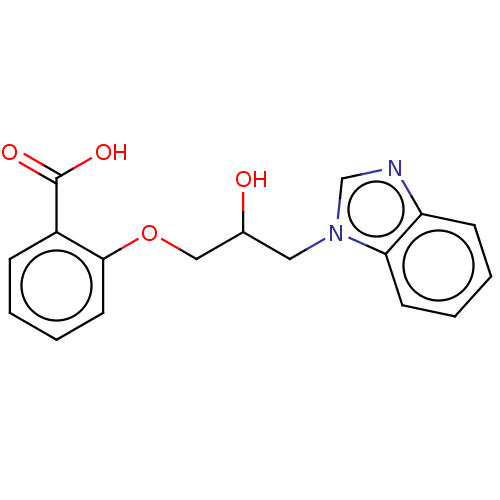 (US10729695, Compound CD-19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453300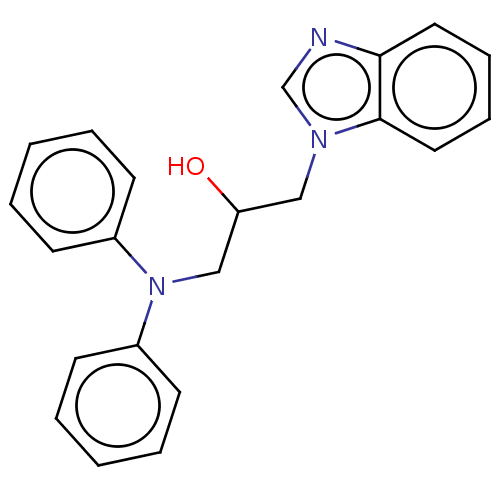 (US10729695, Compound CD-18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453299 (US10729695, Compound CD-16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453298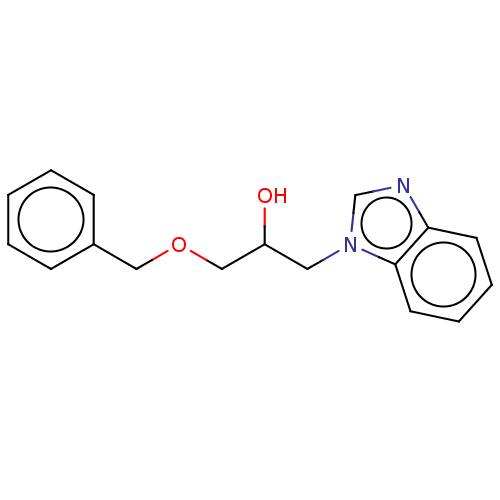 (US10729695, Compound CD-14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453297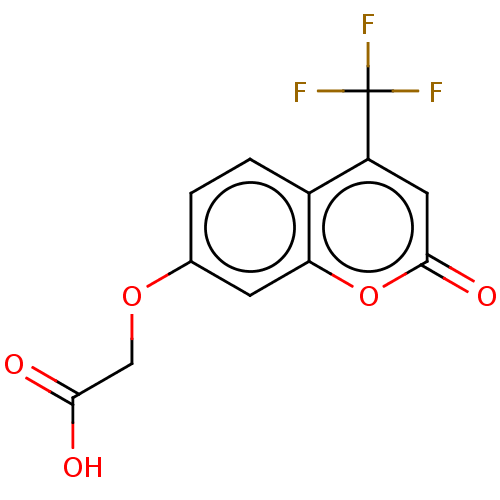 (US10729695, Compound CD-12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453296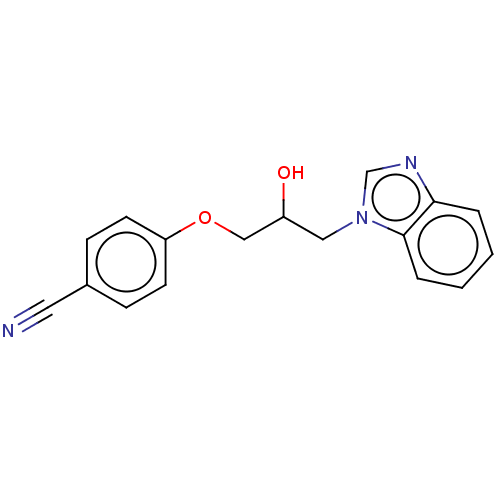 (US10729695, Compound CD-8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453295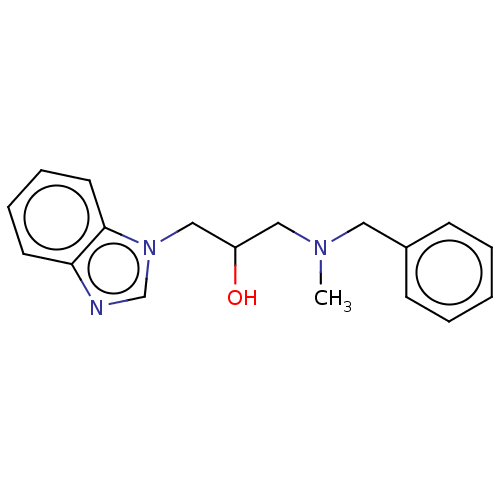 (US10729695, Compound CD-7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453282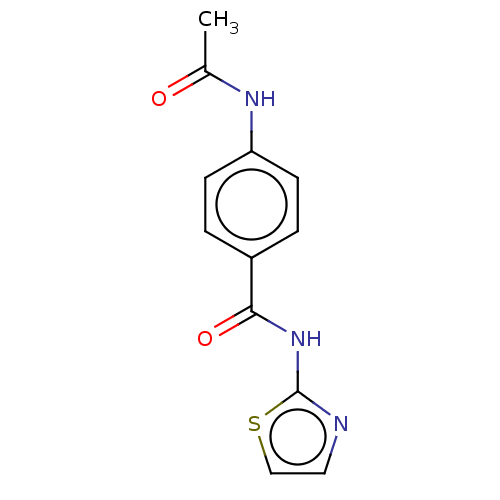 (US10729695, Compound CD-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Advanced glycosylation end product-specific receptor (Homo sapiens (Human)) | BDBM453283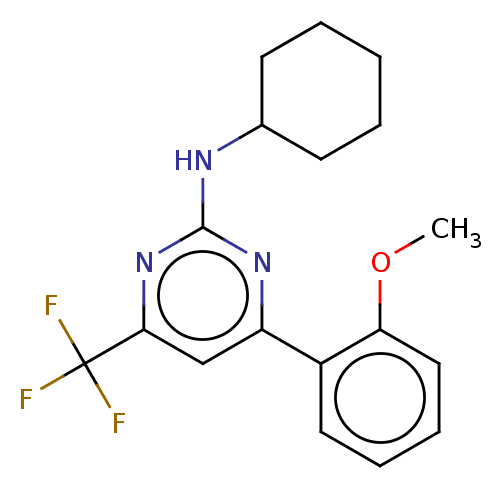 (US10729695, Compound CD-21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
The Research Foundation for the State University of New York; New York University US Patent | Assay Description Day 1Step 1. Add 50 μl anti-mDial (1:160 dilution in 0.1M NaHCO3 pH 9.6)/well. Incubate overnight at 4° C.Day 2Step 2. Use the plate washer to a... | US Patent US10729695 (2020) BindingDB Entry DOI: 10.7270/Q20868CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |