

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM50588805 (CHEMBL5170790) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01170 BindingDB Entry DOI: 10.7270/Q2DZ0D8J | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM31772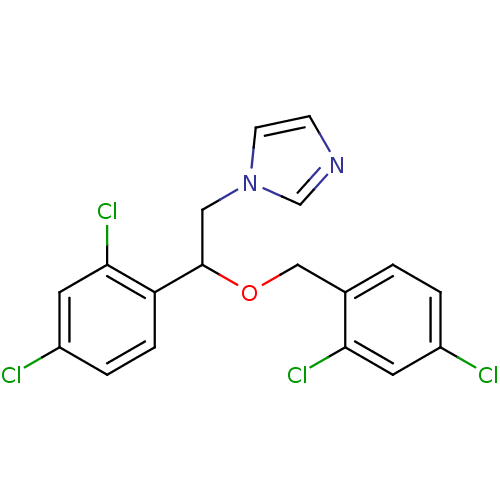 (1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01170 BindingDB Entry DOI: 10.7270/Q2DZ0D8J | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM50588800 (CHEMBL5190388) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01170 BindingDB Entry DOI: 10.7270/Q2DZ0D8J | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM518571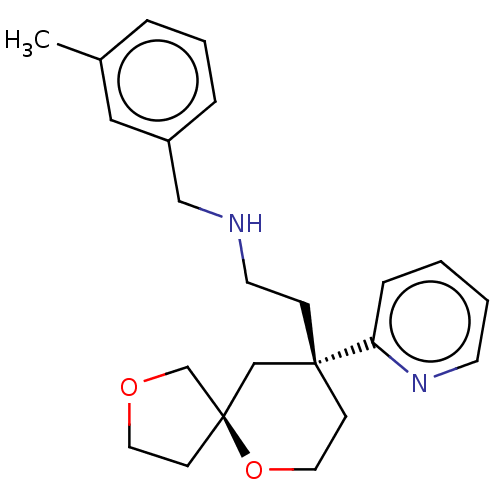 (US11124523, Example (+)-9) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymes | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM518578 (US11124523, Example (+)-16) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymes | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM518580 (US11124523, Example (+)-18) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymes | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM518586 (US11124523, Example (+)-22) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymes | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM518587 (US11124523, Example (+)-23) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymes | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM518593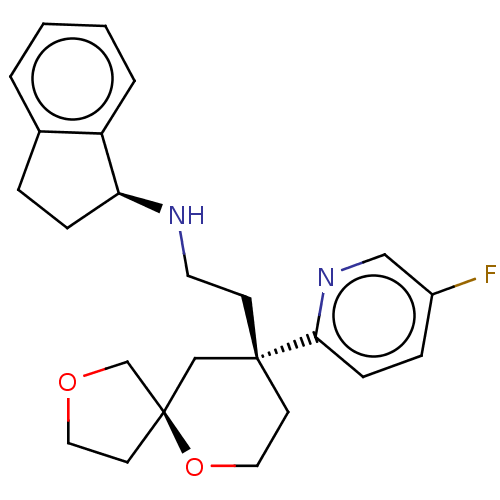 (US11124523, Example (+)-28b) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymes | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM518570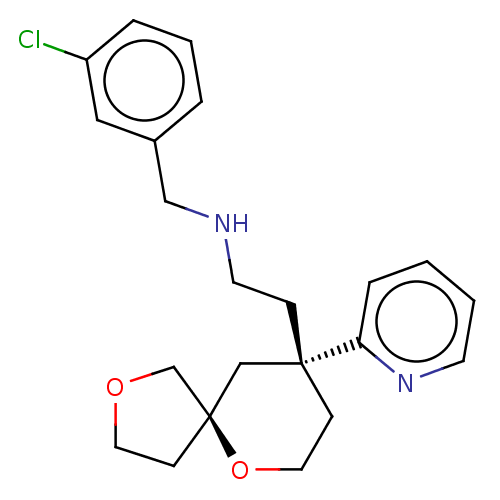 (US11124523, Example (+)-8) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymes | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM518563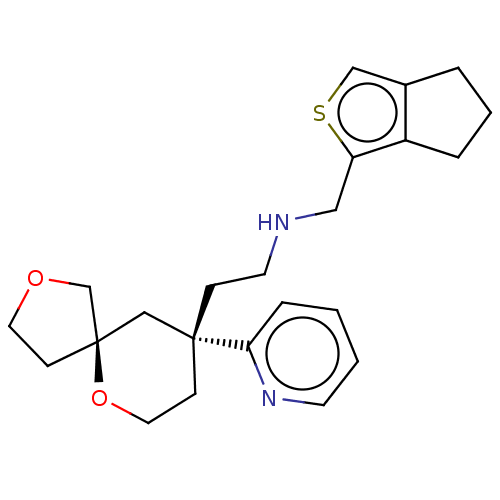 (US11124523, Example (+)-2) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymes | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM518560 (US11124523, Example (+)-1b) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymes | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM50493818 (Oliceridine | TRV-130 | US11124523, Comparative Co...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | US Patent | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To test the inhibitory effects of the compounds of the present disclosure on different isoforms of human cytochrome P450 isoenzymes | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90CTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM50071058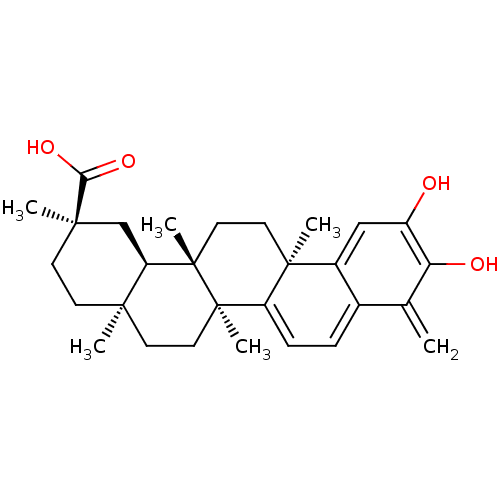 ((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 5.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Rattus norvegicus) | BDBM50426305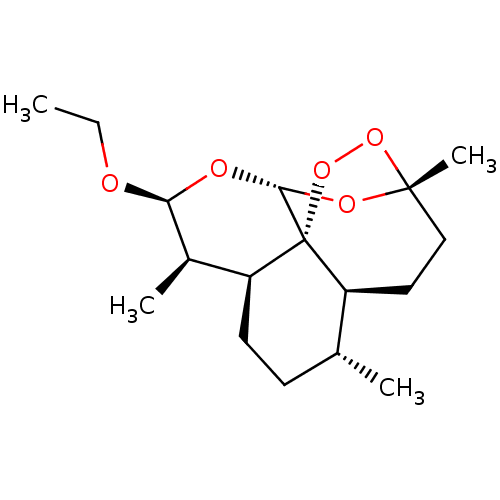 (ARTEMOTIL) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Medicinal& Process Chemistry, Division of Parasitology, Division of Pharmacokinetics and Metabolism, and Sophisticated Analytical Instrument Facility, Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in rat liver microsome using phenacetin as substrate by HPLC-PDA analysis | ACS Med Chem Lett 4: 165-9 (2013) Article DOI: 10.1021/ml300188t BindingDB Entry DOI: 10.7270/Q2V1264R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||