

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor corepressor 1 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins | J Med Chem 56: 6512-20 (2013) Article DOI: 10.1021/jm4008449 BindingDB Entry DOI: 10.7270/Q27S7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor corepressor 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins | J Med Chem 56: 6512-20 (2013) Article DOI: 10.1021/jm4008449 BindingDB Entry DOI: 10.7270/Q27S7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor corepressor 1 (Homo sapiens (Human)) | BDBM50377384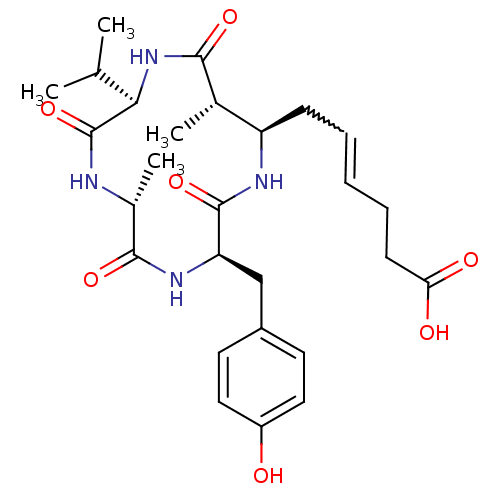 (AZUMAMIDE C) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins | J Med Chem 56: 6512-20 (2013) Article DOI: 10.1021/jm4008449 BindingDB Entry DOI: 10.7270/Q27S7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor corepressor 1 (Homo sapiens (Human)) | BDBM50372469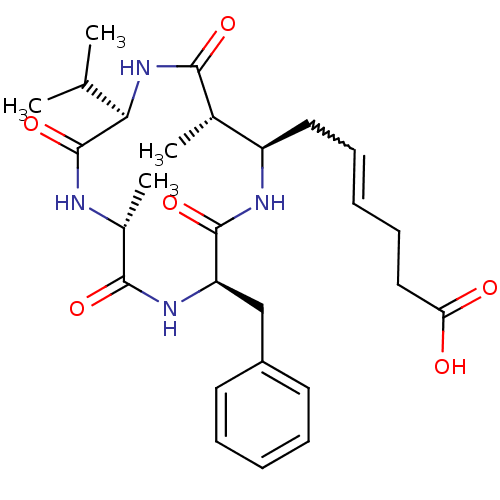 (AZUMAMIDE E) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins | J Med Chem 56: 6512-20 (2013) Article DOI: 10.1021/jm4008449 BindingDB Entry DOI: 10.7270/Q27S7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor corepressor 1 (Homo sapiens (Human)) | BDBM50377383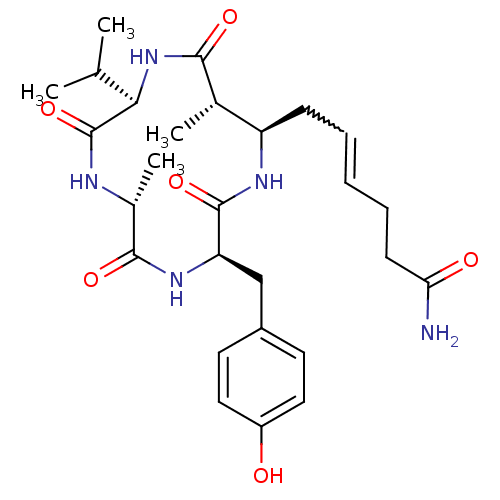 (AZUMAMIDE B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins | J Med Chem 56: 6512-20 (2013) Article DOI: 10.1021/jm4008449 BindingDB Entry DOI: 10.7270/Q27S7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor corepressor 1 (Homo sapiens (Human)) | BDBM50377385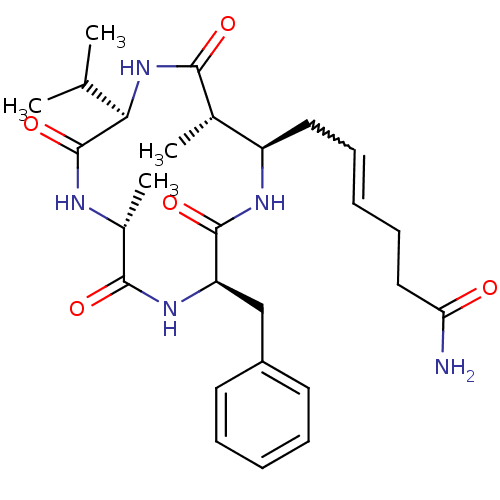 (AZUMAMIDE A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins | J Med Chem 56: 6512-20 (2013) Article DOI: 10.1021/jm4008449 BindingDB Entry DOI: 10.7270/Q27S7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor corepressor 1 (Homo sapiens (Human)) | BDBM50377382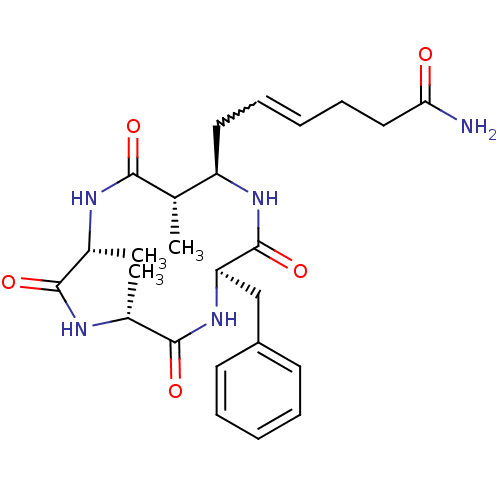 (AZUMAMIDE D) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Denmark Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant human HDAC3/NcoR1 enzyme using flurogenic Ac-LeuGlyLys (Ac)-AMC as substrate after 15 to 30 mins | J Med Chem 56: 6512-20 (2013) Article DOI: 10.1021/jm4008449 BindingDB Entry DOI: 10.7270/Q27S7Q6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||