 Found 132 hits of ki data for polymerid = 50006512,5066
Found 132 hits of ki data for polymerid = 50006512,5066 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122970

(3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-3-yl-furan-2-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1cccnc1 Show InChI InChI=1S/C28H19N3O5/c32-27-18-5-1-2-6-20(18)30-25-19(27)14-31(26(25)16-7-8-22-24(12-16)35-15-34-22)28(33)23-10-9-21(36-23)17-4-3-11-29-13-17/h1-13,26H,14-15H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122969
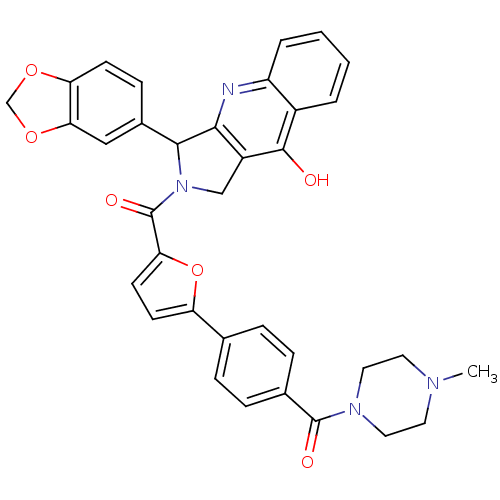
(3-Benzo[1,3]dioxol-5-yl-2-{5-[4-(4-methyl-piperazi...)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C35H30N4O6/c1-37-14-16-38(17-15-37)34(41)22-8-6-21(7-9-22)27-12-13-29(45-27)35(42)39-19-25-31(36-26-5-3-2-4-24(26)33(25)40)32(39)23-10-11-28-30(18-23)44-20-43-28/h2-13,18,32H,14-17,19-20H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50370143
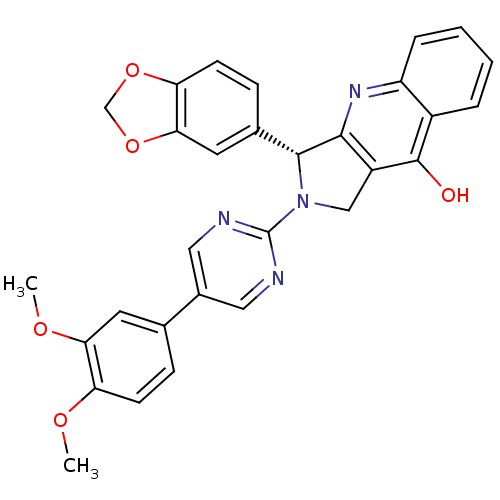
(CHEMBL1744059)Show SMILES COc1ccc(cc1OC)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)[C@H]1c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C30H24N4O5/c1-36-23-9-7-17(11-25(23)37-2)19-13-31-30(32-14-19)34-15-21-27(33-22-6-4-3-5-20(22)29(21)35)28(34)18-8-10-24-26(12-18)39-16-38-24/h3-14,28H,15-16H2,1-2H3,(H,33,35)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118249
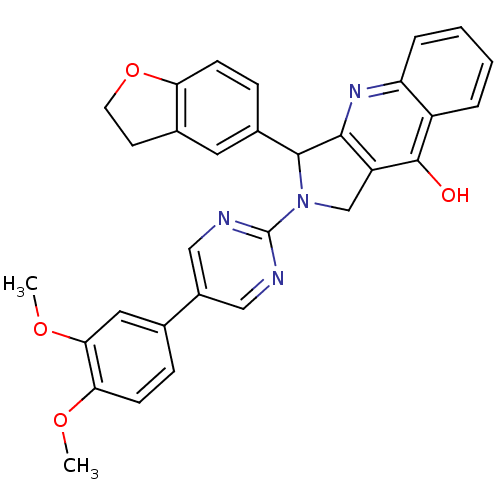
(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(3,4-dimethox...)Show SMILES COc1ccc(cc1OC)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCc2c1 Show InChI InChI=1S/C31H26N4O4/c1-37-26-10-7-18(14-27(26)38-2)21-15-32-31(33-16-21)35-17-23-28(34-24-6-4-3-5-22(24)30(23)36)29(35)20-8-9-25-19(13-20)11-12-39-25/h3-10,13-16,29H,11-12,17H2,1-2H3,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138930
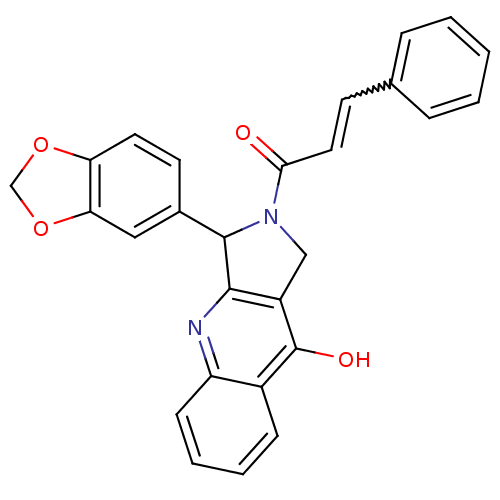
(3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-acryloyl)-1,2,...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)C=Cc1ccccc1 |w:26.31| Show InChI InChI=1S/C27H20N2O4/c30-24(13-10-17-6-2-1-3-7-17)29-15-20-25(28-21-9-5-4-8-19(21)27(20)31)26(29)18-11-12-22-23(14-18)33-16-32-22/h1-14,26H,15-16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118248
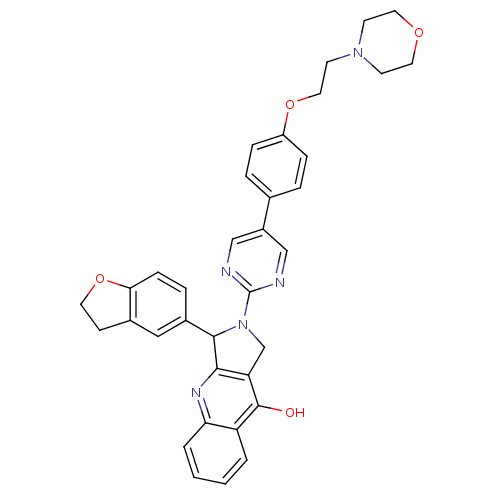
(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)[C@H]1c1ccc2OCCc2c1 Show InChI InChI=1S/C30H24N4O3/c1-36-22-9-6-18(7-10-22)21-15-31-30(32-16-21)34-17-24-27(33-25-5-3-2-4-23(25)29(24)35)28(34)20-8-11-26-19(14-20)12-13-37-26/h2-11,14-16,28H,12-13,17H2,1H3,(H,33,35)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138939
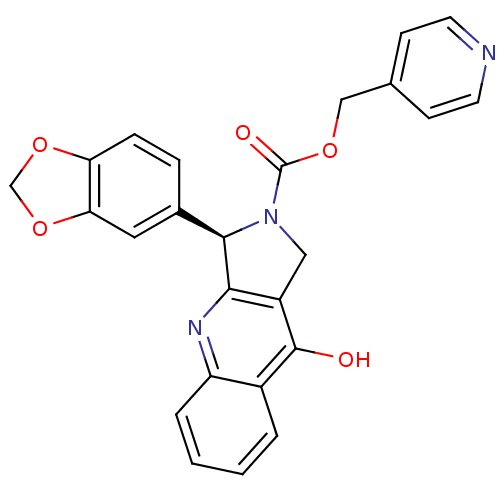
((R)-3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahyd...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccncc1 Show InChI InChI=1S/C25H19N3O5/c29-24-17-3-1-2-4-19(17)27-22-18(24)12-28(25(30)31-13-15-7-9-26-10-8-15)23(22)16-5-6-20-21(11-16)33-14-32-20/h1-11,23H,12-14H2,(H,27,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122990
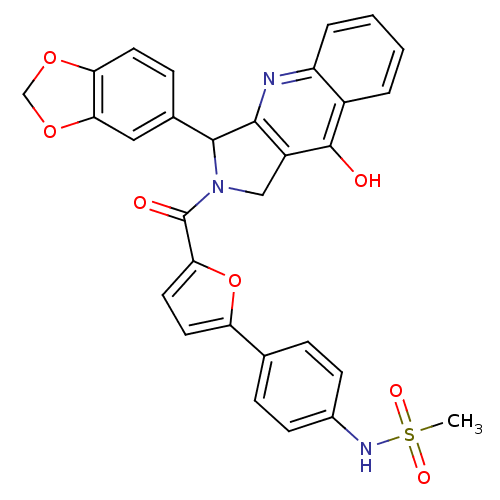
(CHEMBL342159 | N-{4-[5-(3-Benzo[1,3]dioxol-5-yl-9-...)Show SMILES CS(=O)(=O)Nc1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H23N3O7S/c1-41(36,37)32-19-9-6-17(7-10-19)23-12-13-25(40-23)30(35)33-15-21-27(31-22-5-3-2-4-20(22)29(21)34)28(33)18-8-11-24-26(14-18)39-16-38-24/h2-14,28,32H,15-16H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118250
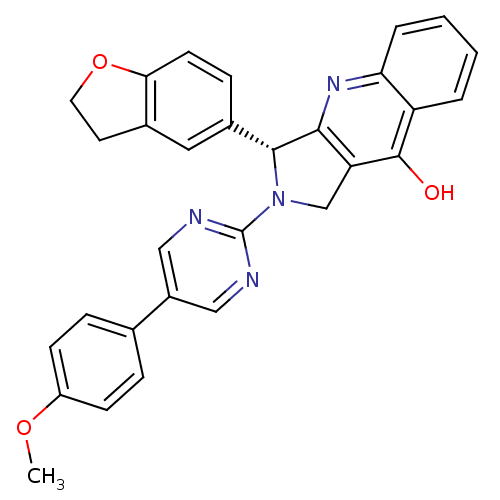
(3-(2,3-Dihydro-benzofuran-5-yl)-2-{5-[4-(2-morphol...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)c1ncc(cn1)-c1ccc(OCCN2CCOCC2)cc1 Show InChI InChI=1S/C35H33N5O4/c41-34-28-3-1-2-4-30(28)38-32-29(34)22-40(33(32)25-7-10-31-24(19-25)11-15-44-31)35-36-20-26(21-37-35)23-5-8-27(9-6-23)43-18-14-39-12-16-42-17-13-39/h1-10,19-21,33H,11-18,22H2,(H,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138929

(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccncc1 Show InChI InChI=1S/C25H19N3O5/c29-24-17-3-1-2-4-19(17)27-22-18(24)12-28(25(30)31-13-15-7-9-26-10-8-15)23(22)16-5-6-20-21(11-16)33-14-32-20/h1-11,23H,12-14H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122974
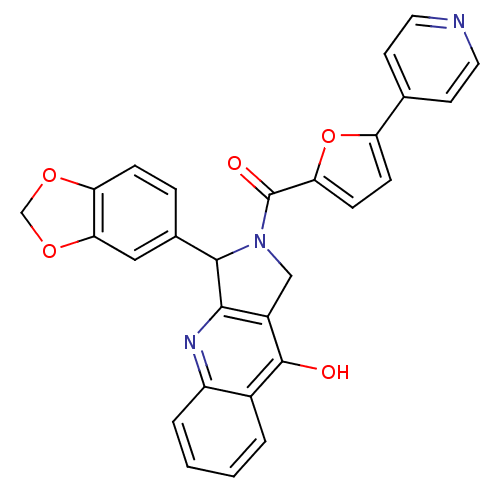
(3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-4-yl-furan-2-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1ccncc1 Show InChI InChI=1S/C28H19N3O5/c32-27-18-3-1-2-4-20(18)30-25-19(27)14-31(26(25)17-5-6-22-24(13-17)35-15-34-22)28(33)23-8-7-21(36-23)16-9-11-29-12-10-16/h1-13,26H,14-15H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118252
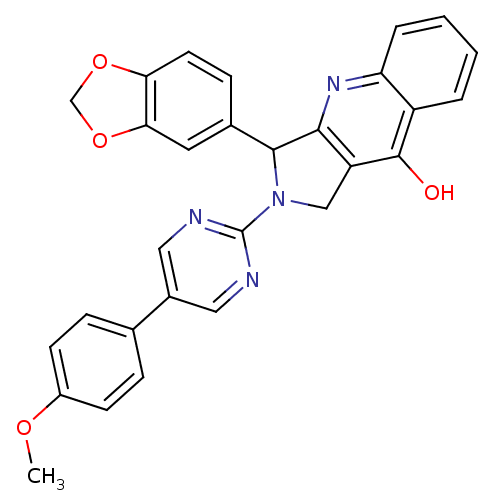
(3-Benzo[1,3]dioxol-5-yl-2-[5-(4-methoxy-phenyl)-py...)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C29H22N4O4/c1-35-20-9-6-17(7-10-20)19-13-30-29(31-14-19)33-15-22-26(32-23-5-3-2-4-21(23)28(22)34)27(33)18-8-11-24-25(12-18)37-16-36-24/h2-14,27H,15-16H2,1H3,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122964

(3-Benzo[1,3]dioxol-5-yl-2-(6-hydroxy-benzofuran-2-...)Show SMILES Oc1ccc2cc(oc2c1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C27H18N2O6/c30-16-7-5-14-9-23(35-21(14)11-16)27(32)29-12-18-24(28-19-4-2-1-3-17(19)26(18)31)25(29)15-6-8-20-22(10-15)34-13-33-20/h1-11,25,30H,12-13H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138936
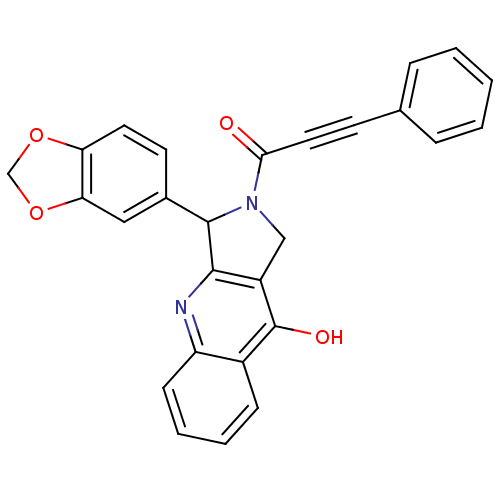
(3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-propynoyl)-1,2...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)C#Cc1ccccc1 Show InChI InChI=1S/C27H18N2O4/c30-24(13-10-17-6-2-1-3-7-17)29-15-20-25(28-21-9-5-4-8-19(21)27(20)31)26(29)18-11-12-22-23(14-18)33-16-32-22/h1-9,11-12,14,26H,15-16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118255
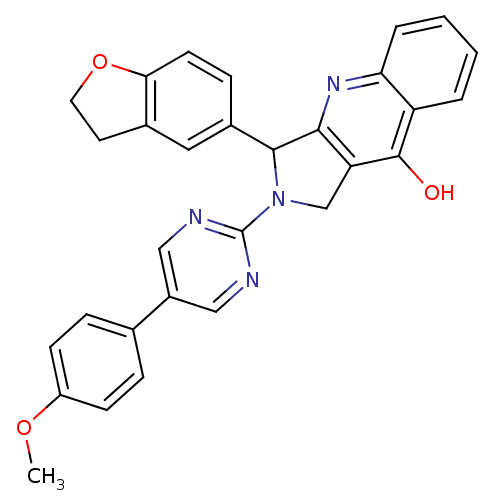
(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCc2c1 Show InChI InChI=1S/C30H24N4O3/c1-36-22-9-6-18(7-10-22)21-15-31-30(32-16-21)34-17-24-27(33-25-5-3-2-4-23(25)29(24)35)28(34)20-8-11-26-19(14-20)12-13-37-26/h2-11,14-16,28H,12-13,17H2,1H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122966
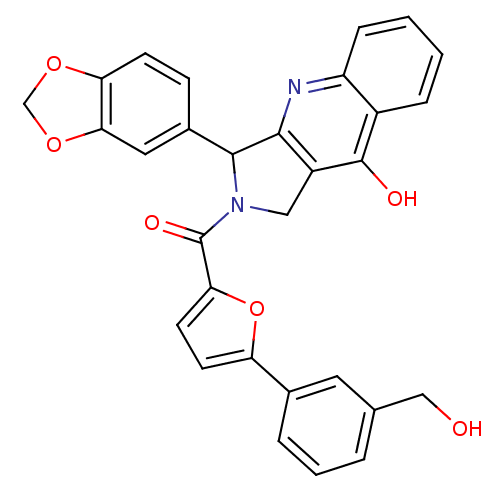
(3-Benzo[1,3]dioxol-5-yl-2-[5-(3-hydroxymethyl-phen...)Show SMILES OCc1cccc(c1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H22N2O6/c33-15-17-4-3-5-18(12-17)23-10-11-25(38-23)30(35)32-14-21-27(31-22-7-2-1-6-20(22)29(21)34)28(32)19-8-9-24-26(13-19)37-16-36-24/h1-13,28,33H,14-16H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118251
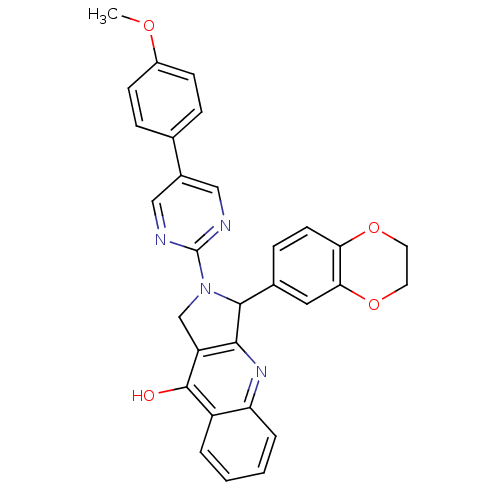
(3-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-2-[5-(4-meth...)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCOc2c1 Show InChI InChI=1S/C30H24N4O4/c1-36-21-9-6-18(7-10-21)20-15-31-30(32-16-20)34-17-23-27(33-24-5-3-2-4-22(24)29(23)35)28(34)19-8-11-25-26(14-19)38-13-12-37-25/h2-11,14-16,28H,12-13,17H2,1H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122971
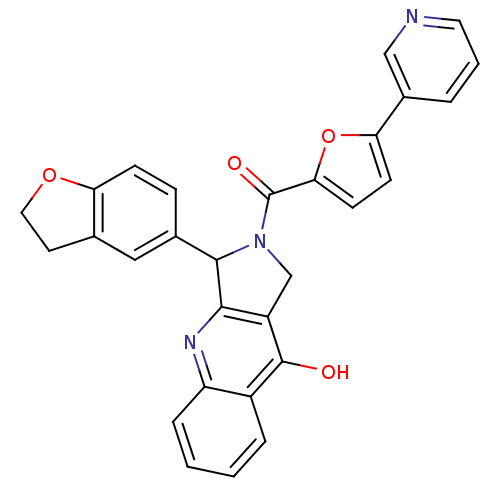
(3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-3-yl-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)C(=O)c1ccc(o1)-c1cccnc1 Show InChI InChI=1S/C29H21N3O4/c33-28-20-5-1-2-6-22(20)31-26-21(28)16-32(27(26)18-7-8-23-17(14-18)11-13-35-23)29(34)25-10-9-24(36-25)19-4-3-12-30-15-19/h1-10,12,14-15,27H,11,13,16H2,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118258
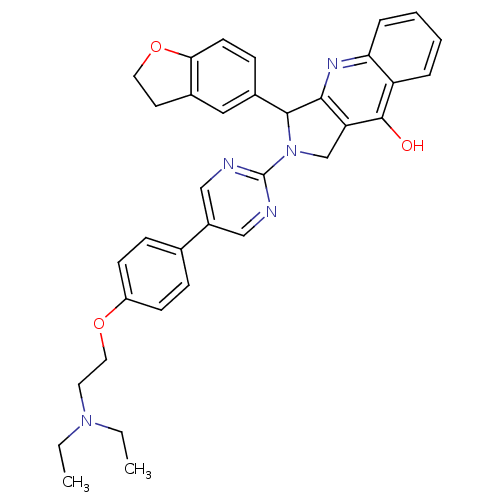
(2-{5-[4-(2-Diethylamino-ethoxy)-phenyl]-pyrimidin-...)Show SMILES CCN(CC)CCOc1ccc(cc1)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCc2c1 Show InChI InChI=1S/C35H35N5O3/c1-3-39(4-2)16-18-42-27-12-9-23(10-13-27)26-20-36-35(37-21-26)40-22-29-32(38-30-8-6-5-7-28(30)34(29)41)33(40)25-11-14-31-24(19-25)15-17-43-31/h5-14,19-21,33H,3-4,15-18,22H2,1-2H3,(H,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138927
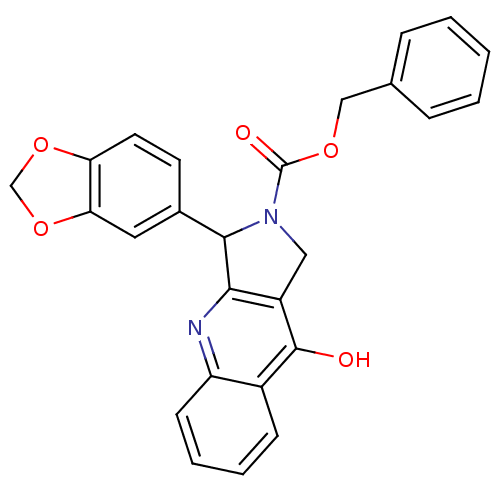
(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H20N2O5/c29-25-18-8-4-5-9-20(18)27-23-19(25)13-28(26(30)31-14-16-6-2-1-3-7-16)24(23)17-10-11-21-22(12-17)33-15-32-21/h1-12,24H,13-15H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122983

(3-Benzo[1,3]dioxol-5-yl-2-[5-(4-nitro-phenyl)-fura...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C29H19N3O7/c33-28-19-3-1-2-4-21(19)30-26-20(28)14-31(27(26)17-7-10-23-25(13-17)38-15-37-23)29(34)24-12-11-22(39-24)16-5-8-18(9-6-16)32(35)36/h1-13,27H,14-15H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122973

(3-Benzo[1,3]dioxol-5-yl-2-[5-(4-hydroxymethyl-phen...)Show SMILES OCc1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H22N2O6/c33-15-17-5-7-18(8-6-17)23-11-12-25(38-23)30(35)32-14-21-27(31-22-4-2-1-3-20(22)29(21)34)28(32)19-9-10-24-26(13-19)37-16-36-24/h1-13,28,33H,14-16H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50088373
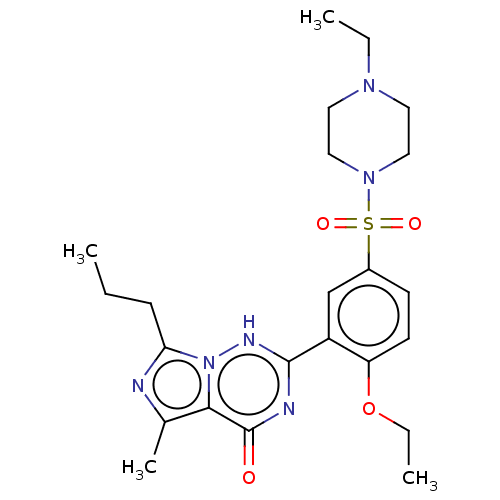
(CHEBI:46295 | Vardenafil | cid_110634)Show SMILES CCCc1nc(C)c2n1[nH]c(nc2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122980
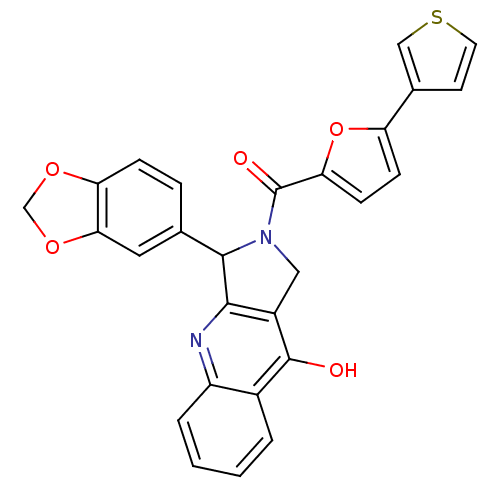
(3-Benzo[1,3]dioxol-5-yl-2-(5-thiophen-3-yl-furan-2...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1ccsc1 Show InChI InChI=1S/C27H18N2O5S/c30-26-17-3-1-2-4-19(17)28-24-18(26)12-29(25(24)15-5-6-21-23(11-15)33-14-32-21)27(31)22-8-7-20(34-22)16-9-10-35-13-16/h1-11,13,25H,12,14H2,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122981
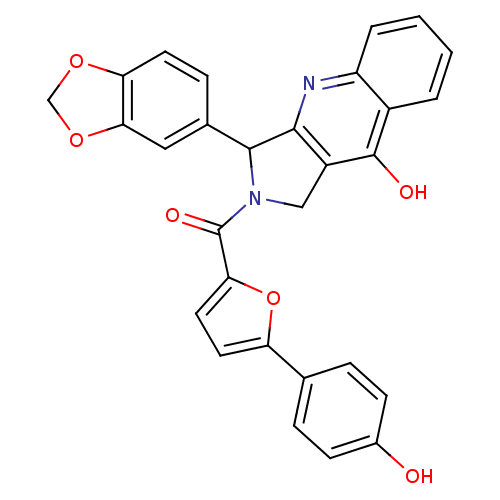
(3-Benzo[1,3]dioxol-5-yl-2-[5-(4-hydroxy-phenyl)-fu...)Show SMILES Oc1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C29H20N2O6/c32-18-8-5-16(6-9-18)22-11-12-24(37-22)29(34)31-14-20-26(30-21-4-2-1-3-19(21)28(20)33)27(31)17-7-10-23-25(13-17)36-15-35-23/h1-13,27,32H,14-15H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122987
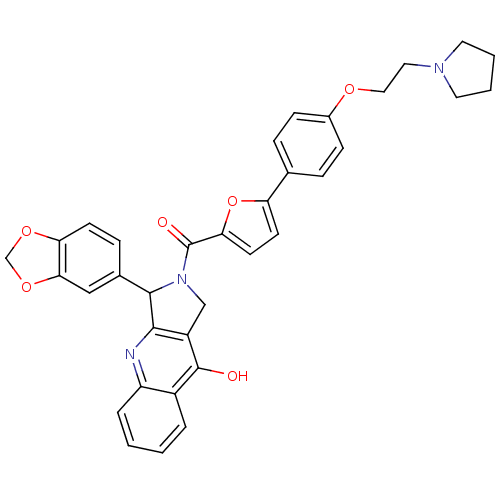
(3-Benzo[1,3]dioxol-5-yl-2-{5-[4-(2-pyrrolidin-1-yl...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C35H31N3O6/c39-34-25-5-1-2-6-27(25)36-32-26(34)20-38(33(32)23-9-12-29-31(19-23)43-21-42-29)35(40)30-14-13-28(44-30)22-7-10-24(11-8-22)41-18-17-37-15-3-4-16-37/h1-2,5-14,19,33H,3-4,15-18,20-21H2,(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122989
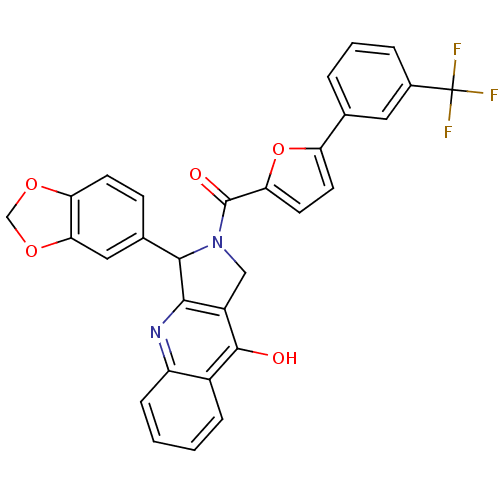
(3-Benzo[1,3]dioxol-5-yl-2-[5-(3-trifluoromethyl-ph...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C30H19F3N2O5/c31-30(32,33)18-5-3-4-16(12-18)22-10-11-24(40-22)29(37)35-14-20-26(34-21-7-2-1-6-19(21)28(20)36)27(35)17-8-9-23-25(13-17)39-15-38-23/h1-13,27H,14-15H2,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122967

(4-[5-(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrah...)Show SMILES COC(=O)c1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C31H22N2O7/c1-37-31(36)18-8-6-17(7-9-18)23-12-13-25(40-23)30(35)33-15-21-27(32-22-5-3-2-4-20(22)29(21)34)28(33)19-10-11-24-26(14-19)39-16-38-24/h2-14,28H,15-16H2,1H3,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14776

(2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...)Show SMILES CCCc1nc(C)c2n1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human phosphodiesterase 5 |
J Med Chem 48: 3449-62 (2005)
Article DOI: 10.1021/jm040217u
BindingDB Entry DOI: 10.7270/Q21G0N2H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122976
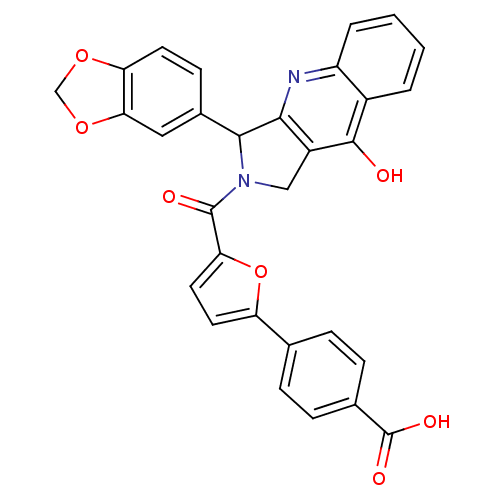
(4-[5-(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrah...)Show SMILES OC(=O)c1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H20N2O7/c33-28-19-3-1-2-4-21(19)31-26-20(28)14-32(27(26)18-9-10-23-25(13-18)38-15-37-23)29(34)24-12-11-22(39-24)16-5-7-17(8-6-16)30(35)36/h1-13,27H,14-15H2,(H,31,33)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118256
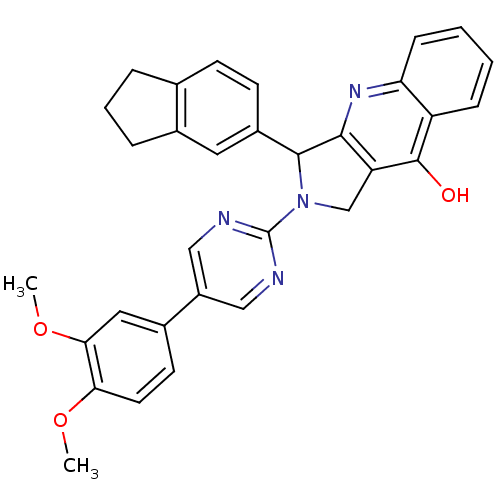
(2-[5-(3,4-Dimethoxy-phenyl)-pyrimidin-2-yl]-3-inda...)Show SMILES COc1ccc(cc1OC)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2CCCc2c1 Show InChI InChI=1S/C32H28N4O3/c1-38-27-13-12-21(15-28(27)39-2)23-16-33-32(34-17-23)36-18-25-29(35-26-9-4-3-8-24(26)31(25)37)30(36)22-11-10-19-6-5-7-20(19)14-22/h3-4,8-17,30H,5-7,18H2,1-2H3,(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138935

(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C26H21N3O4/c30-25-18-8-4-5-9-20(18)28-23-19(25)14-29(26(31)27-13-16-6-2-1-3-7-16)24(23)17-10-11-21-22(12-17)33-15-32-21/h1-12,24H,13-15H2,(H,27,31)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118257

(3-(2,3-Dihydro-benzofuran-5-yl)-2-pyrimidin-2-yl-1...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)c1ncccn1 Show InChI InChI=1S/C23H18N4O2/c28-22-16-4-1-2-5-18(16)26-20-17(22)13-27(23-24-9-3-10-25-23)21(20)15-6-7-19-14(12-15)8-11-29-19/h1-7,9-10,12,21H,8,11,13H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118254
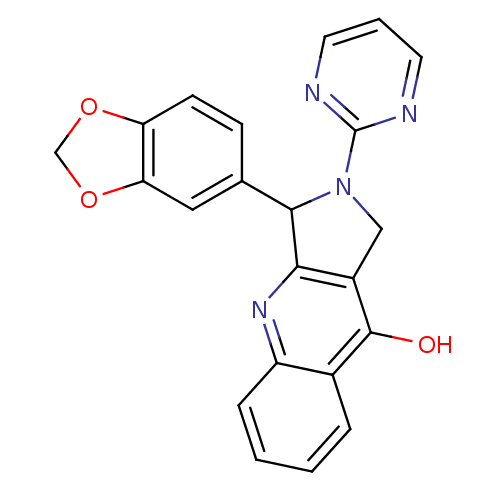
(3-Benzo[1,3]dioxol-5-yl-2-pyrimidin-2-yl-1,2,3,4-t...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)c1ncccn1 Show InChI InChI=1S/C22H16N4O3/c27-21-14-4-1-2-5-16(14)25-19-15(21)11-26(22-23-8-3-9-24-22)20(19)13-6-7-17-18(10-13)29-12-28-17/h1-10,20H,11-12H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122982
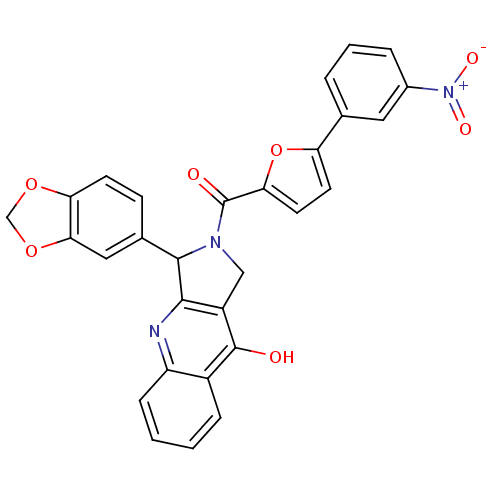
(3-Benzo[1,3]dioxol-5-yl-2-[5-(3-nitro-phenyl)-fura...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C29H19N3O7/c33-28-19-6-1-2-7-21(19)30-26-20(28)14-31(27(26)17-8-9-23-25(13-17)38-15-37-23)29(34)24-11-10-22(39-24)16-4-3-5-18(12-16)32(35)36/h1-13,27H,14-15H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) |
Eur J Med Chem 158: 767-780 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.028
BindingDB Entry DOI: 10.7270/Q2JS9T4N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122985
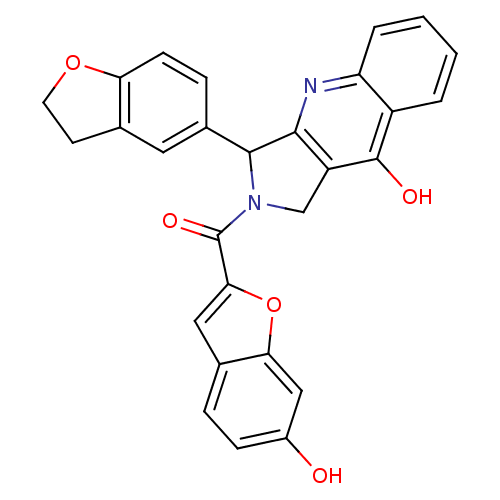
(3-(2,3-Dihydro-benzofuran-5-yl)-2-(6-hydroxy-benzo...)Show SMILES Oc1ccc2cc(oc2c1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCc2c1 Show InChI InChI=1S/C28H20N2O5/c31-18-7-5-15-12-24(35-23(15)13-18)28(33)30-14-20-25(29-21-4-2-1-3-19(21)27(20)32)26(30)17-6-8-22-16(11-17)9-10-34-22/h1-8,11-13,26,31H,9-10,14H2,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122988

(3-Benzo[1,3]dioxol-5-yl-2-[5-(4-methoxy-phenyl)-fu...)Show SMILES COc1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H22N2O6/c1-35-19-9-6-17(7-10-19)23-12-13-25(38-23)30(34)32-15-21-27(31-22-5-3-2-4-20(22)29(21)33)28(32)18-8-11-24-26(14-18)37-16-36-24/h2-14,28H,15-16H2,1H3,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122986
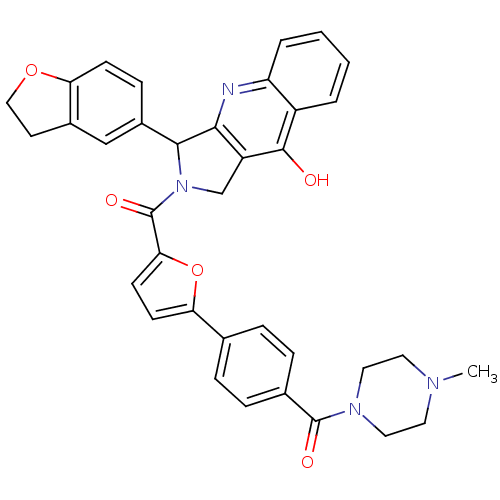
(3-(2,3-Dihydro-benzofuran-5-yl)-2-{5-[4-(4-methyl-...)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCc2c1 Show InChI InChI=1S/C36H32N4O5/c1-38-15-17-39(18-16-38)35(42)23-8-6-22(7-9-23)30-12-13-31(45-30)36(43)40-21-27-32(37-28-5-3-2-4-26(28)34(27)41)33(40)25-10-11-29-24(20-25)14-19-44-29/h2-13,20,33H,14-19,21H2,1H3,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118247

(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-hydroxy-ph...)Show SMILES Oc1ccc(cc1)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCc2c1 Show InChI InChI=1S/C29H22N4O3/c34-21-8-5-17(6-9-21)20-14-30-29(31-15-20)33-16-23-26(32-24-4-2-1-3-22(24)28(23)35)27(33)19-7-10-25-18(13-19)11-12-36-25/h1-10,13-15,27,34H,11-12,16H2,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE5 from human corpus cavernosum |
Bioorg Med Chem Lett 13: 761-5 (2003)
BindingDB Entry DOI: 10.7270/Q29886CM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122984
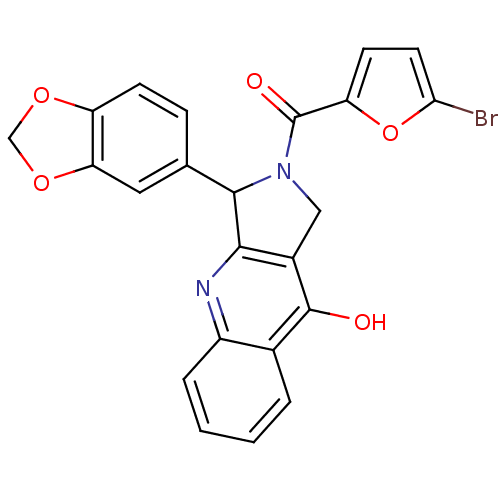
(3-Benzo[1,3]dioxol-5-yl-2-(5-bromo-furan-2-carbony...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(Br)o1 Show InChI InChI=1S/C23H15BrN2O5/c24-19-8-7-17(31-19)23(28)26-10-14-20(25-15-4-2-1-3-13(15)22(14)27)21(26)12-5-6-16-18(9-12)30-11-29-16/h1-9,21H,10-11H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122978
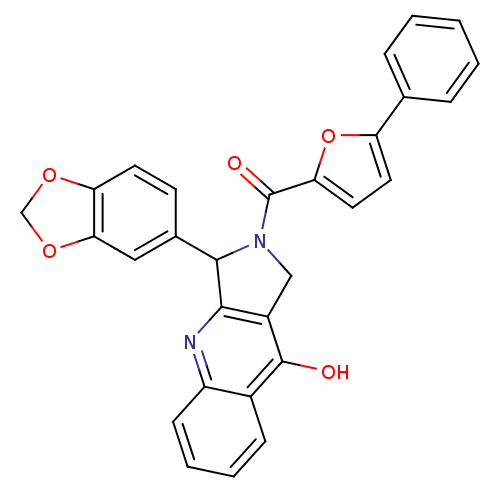
(3-Benzo[1,3]dioxol-5-yl-2-(5-phenyl-furan-2-carbon...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1ccccc1 Show InChI InChI=1S/C29H20N2O5/c32-28-19-8-4-5-9-21(19)30-26-20(28)15-31(27(26)18-10-11-23-25(14-18)35-16-34-23)29(33)24-13-12-22(36-24)17-6-2-1-3-7-17/h1-14,27H,15-16H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122979
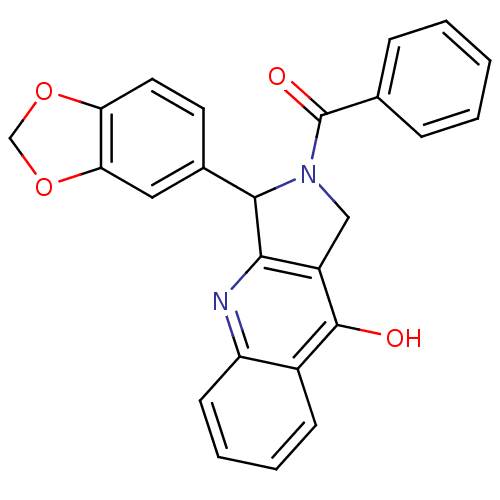
(3-Benzo[1,3]dioxol-5-yl-2-benzoyl-1,2,3,4-tetrahyd...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H18N2O4/c28-24-17-8-4-5-9-19(17)26-22-18(24)13-27(25(29)15-6-2-1-3-7-15)23(22)16-10-11-20-21(12-16)31-14-30-20/h1-12,23H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122979

(3-Benzo[1,3]dioxol-5-yl-2-benzoyl-1,2,3,4-tetrahyd...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H18N2O4/c28-24-17-8-4-5-9-19(17)26-22-18(24)13-27(25(29)15-6-2-1-3-7-15)23(22)16-10-11-20-21(12-16)31-14-30-20/h1-12,23H,13-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122975
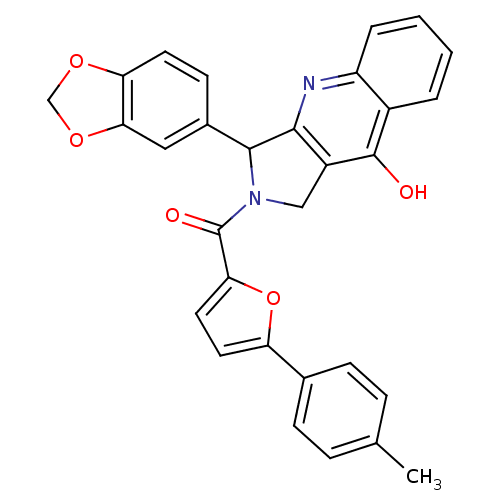
(3-Benzo[1,3]dioxol-5-yl-2-(5-p-tolyl-furan-2-carbo...)Show SMILES Cc1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H22N2O5/c1-17-6-8-18(9-7-17)23-12-13-25(37-23)30(34)32-15-21-27(31-22-5-3-2-4-20(22)29(21)33)28(32)19-10-11-24-26(14-19)36-16-35-24/h2-14,28H,15-16H2,1H3,(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122968
(4-[5-(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrah...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1ccc(C=O)cc1 Show InChI InChI=1S/C30H20N2O6/c33-15-17-5-7-18(8-6-17)23-11-12-25(38-23)30(35)32-14-21-27(31-22-4-2-1-3-20(22)29(21)34)28(32)19-9-10-24-26(13-19)37-16-36-24/h1-13,15,28H,14,16H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data