

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50512103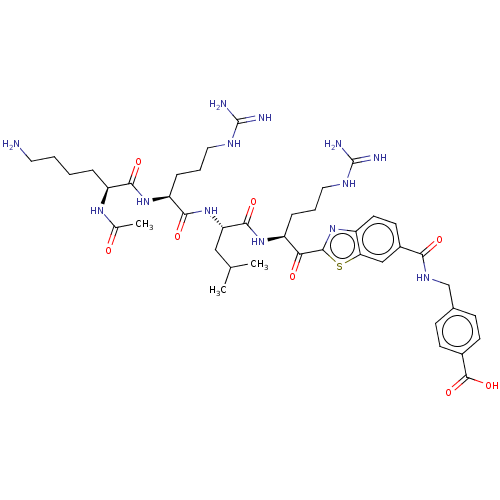 (CHEMBL4464524) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Pernambuco Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA serine protease domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition by fluores... | Eur J Med Chem 170: 237-260 (2019) Article DOI: 10.1016/j.ejmech.2019.03.024 BindingDB Entry DOI: 10.7270/Q2X92FNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50512105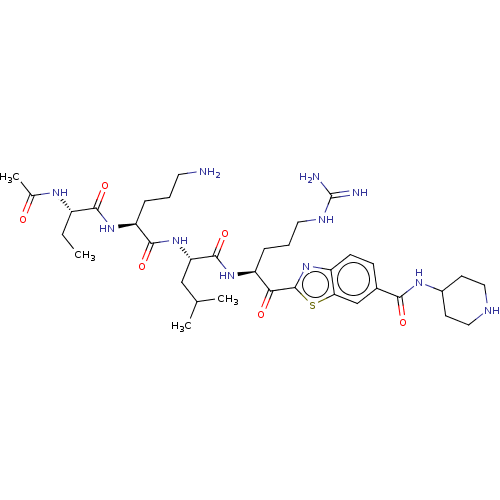 (CHEMBL4587327) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal de Pernambuco Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA serine protease domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition by fluores... | Eur J Med Chem 170: 237-260 (2019) Article DOI: 10.1016/j.ejmech.2019.03.024 BindingDB Entry DOI: 10.7270/Q2X92FNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032991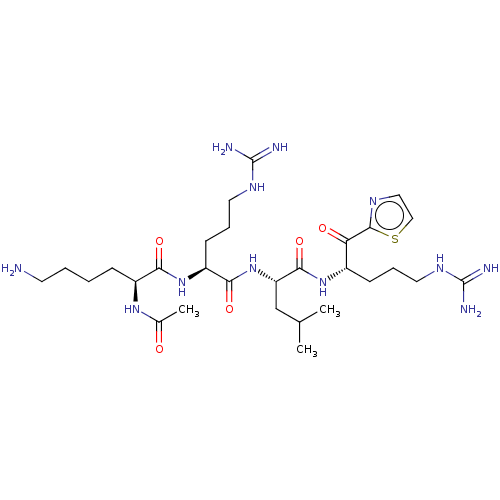 (CHEMBL3356596) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032947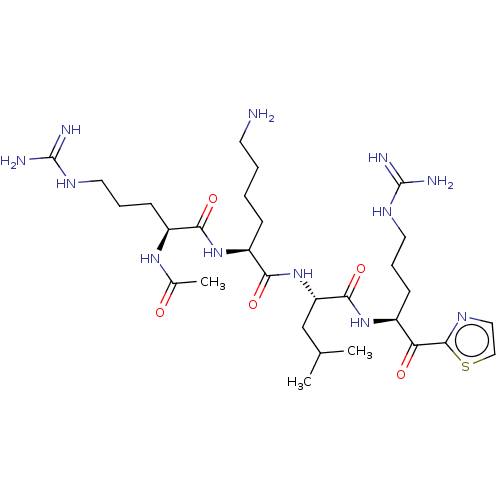 (CHEMBL3356606) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032999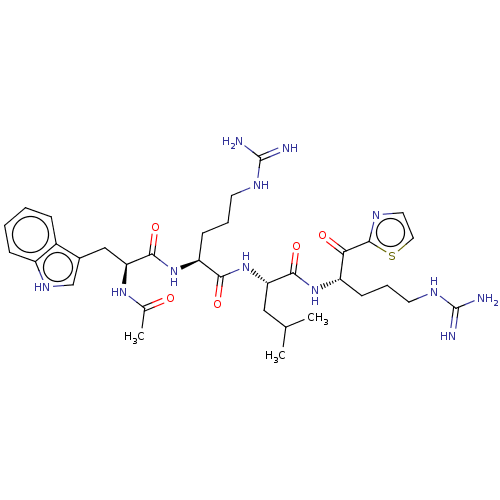 (CHEMBL3356597) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032944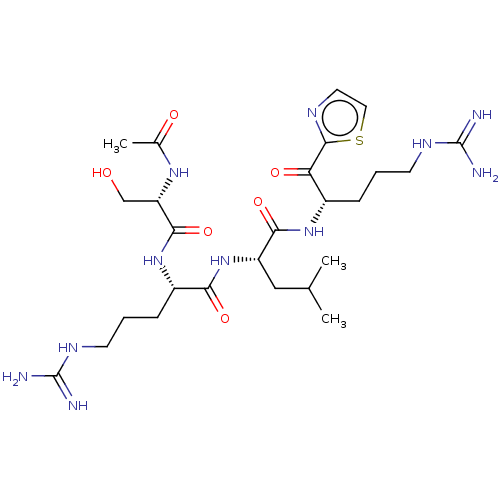 (CHEMBL3356603) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged HGFA (unknown origin) expressed in baculovirus-infected Sf9 cells using Boc-QLR-AMC as substrate incu... | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50063698 (4-Guanidino-benzoic acid 6-carbamimidoyl-naphthale...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518599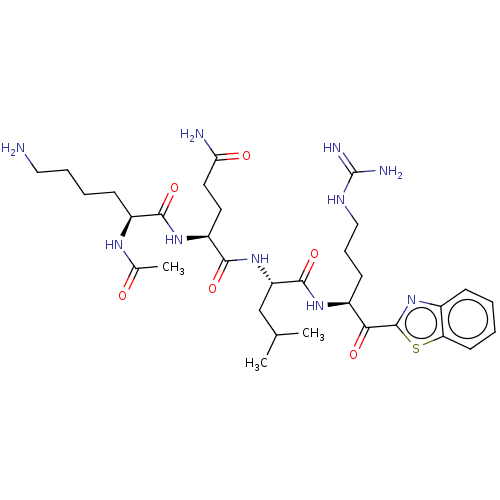 (CHEMBL4454016) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518598 (CHEMBL4540950) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032936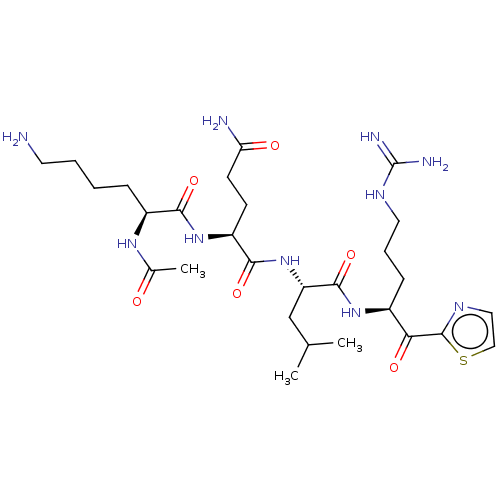 (CHEMBL3356589) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032941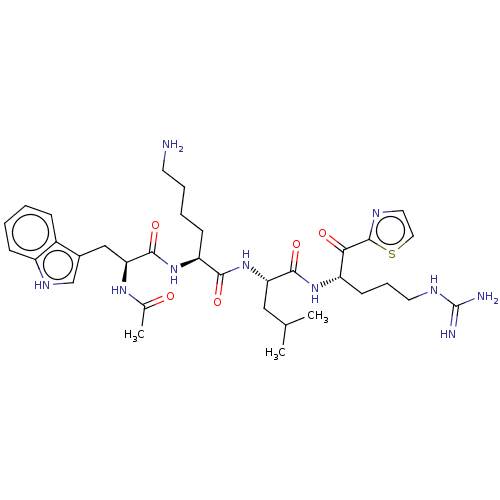 (CHEMBL3356600) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032942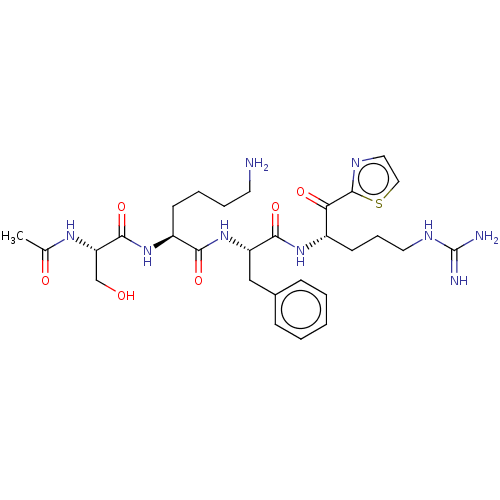 (CHEMBL3356601) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032933 (CHEMBL3356592) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032931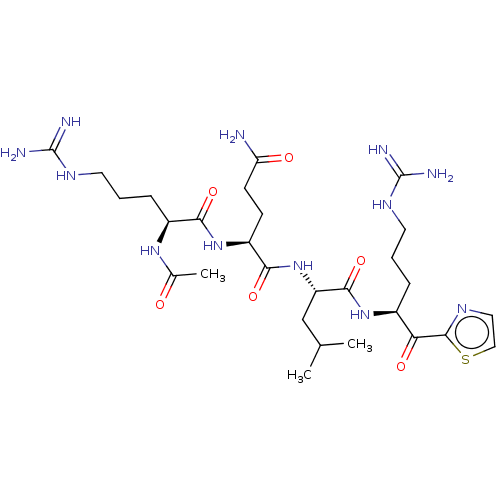 (CHEMBL3356594) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032946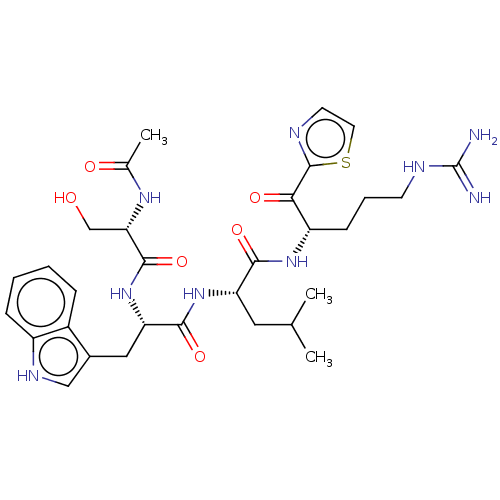 (CHEMBL3356605) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032934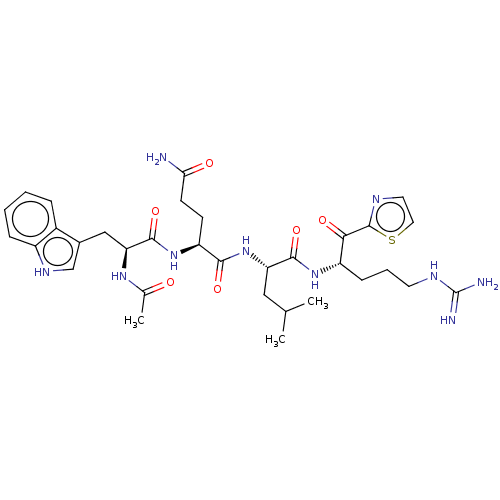 (CHEMBL3356591) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032943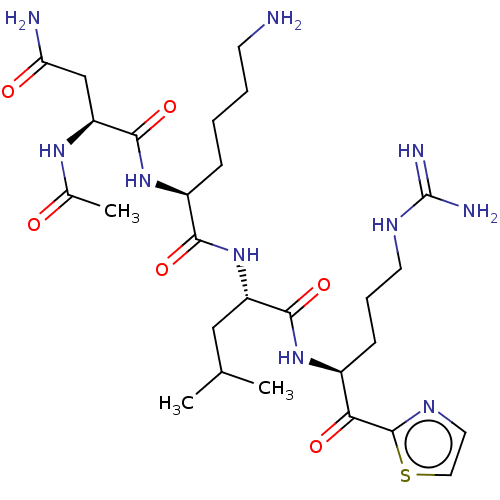 (CHEMBL3356602) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032932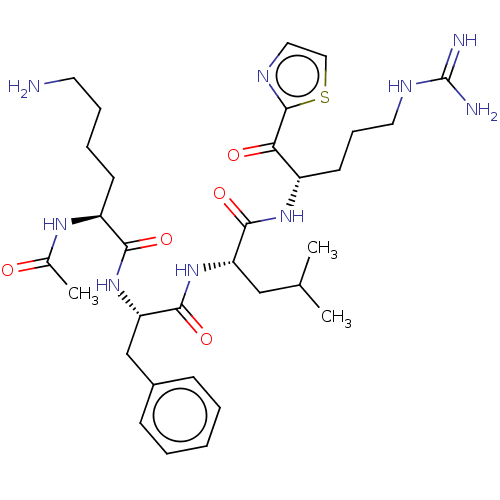 (CHEMBL3356593) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032939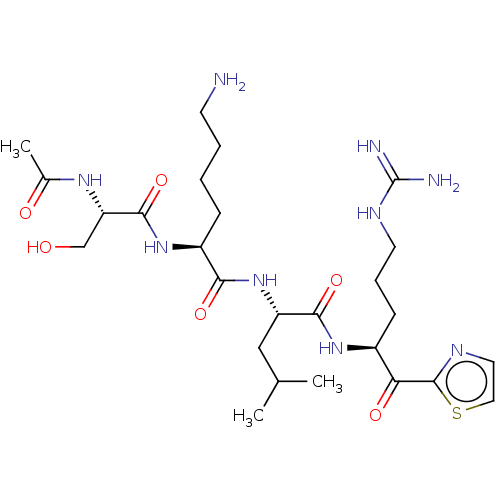 (CHEMBL3356598) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032935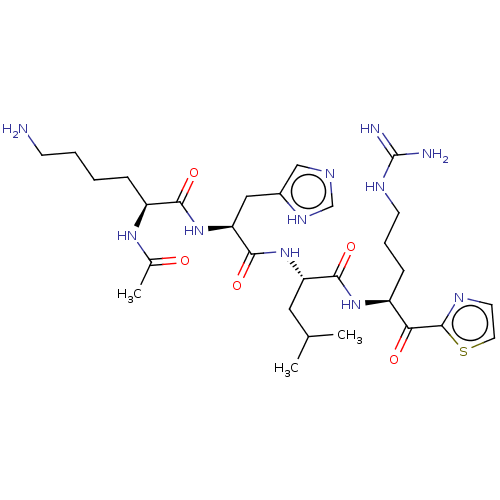 (CHEMBL3356590) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032945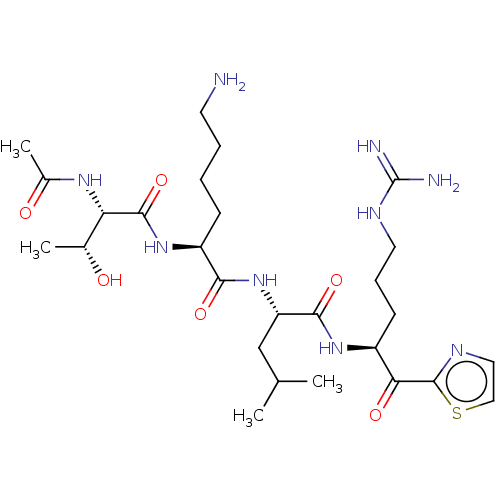 (CHEMBL3356604) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158157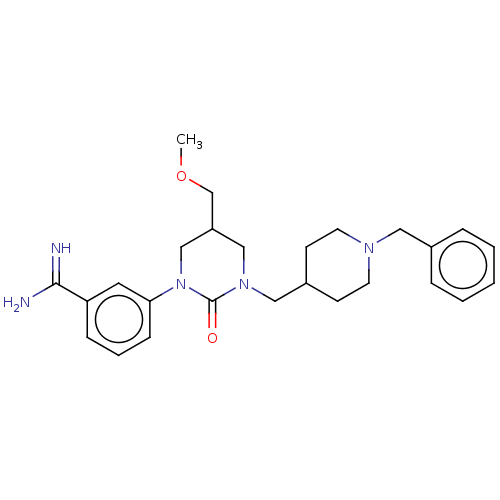 (CHEMBL3780434) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032930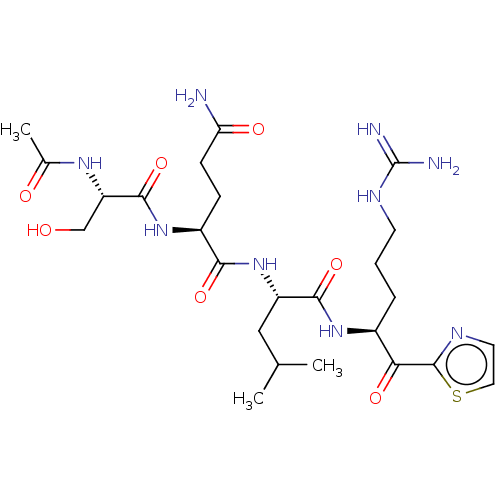 (CHEMBL3356595) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50286441 ((S)-2-((S)-2-Acetylamino-4-(S)-methyl-pentanoylami...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158155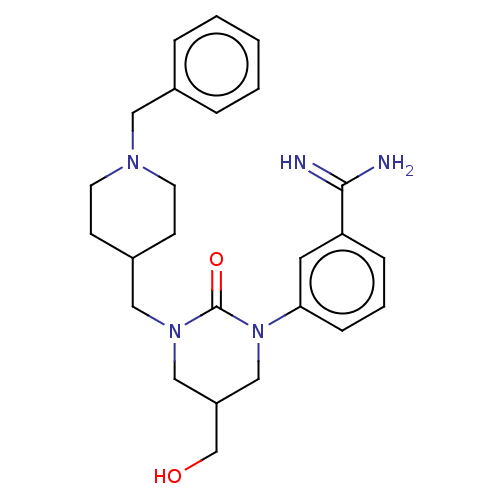 (CHEMBL3781354) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50032940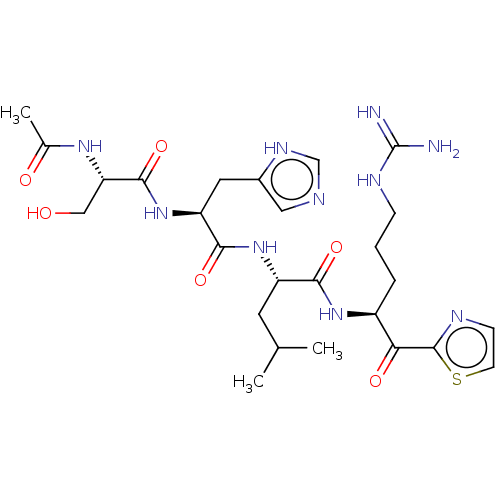 (CHEMBL3356599) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 332 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant HGFA (unknown origin) using Boc-QLR-AMC as substrate by chromogenic proteolytic assay | ACS Med Chem Lett 5: 1219-24 (2014) Article DOI: 10.1021/ml500254r BindingDB Entry DOI: 10.7270/Q2VM4DV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158151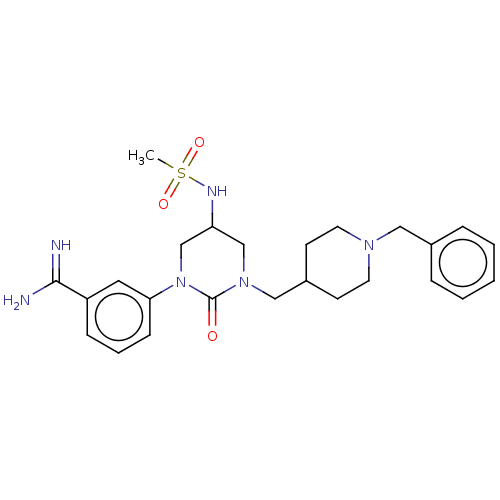 (CHEMBL3780273) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158153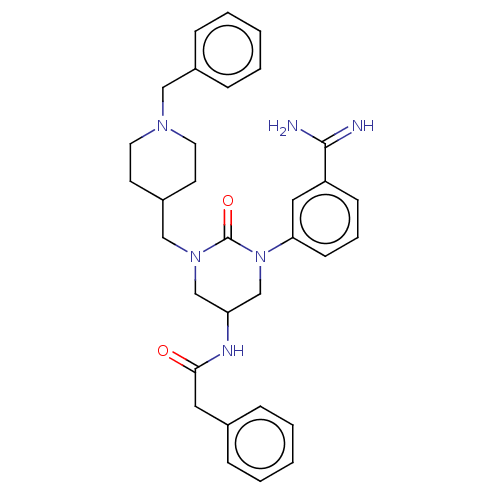 (CHEMBL3780548) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158159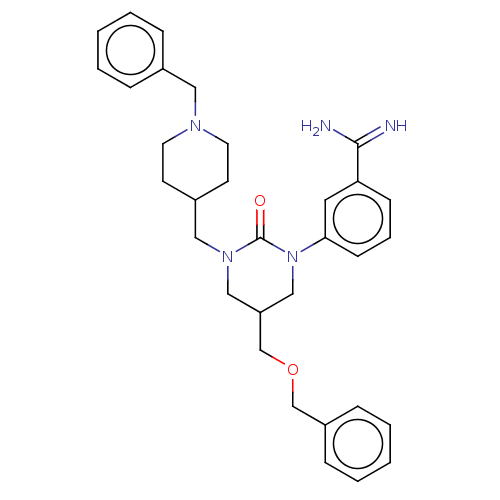 (CHEMBL3780036) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158101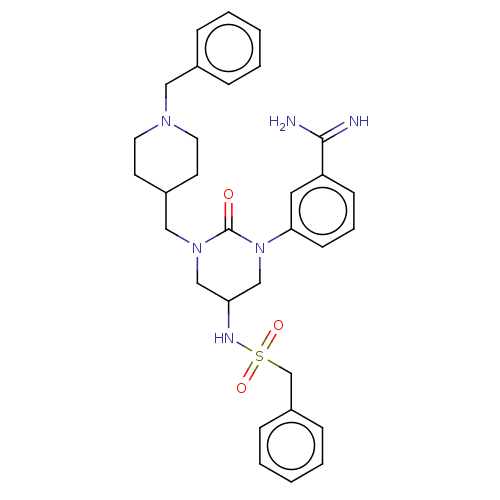 (CHEMBL3781673) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518608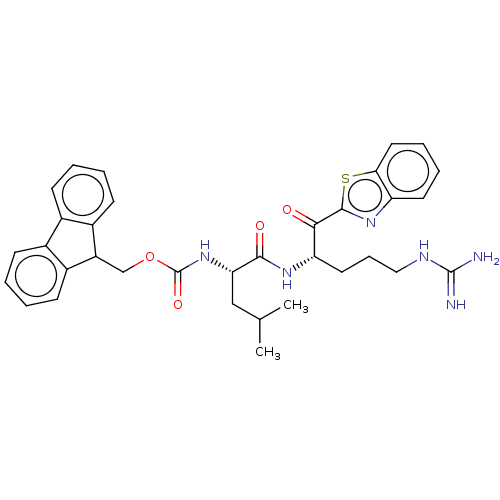 (CHEMBL4561858) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50157632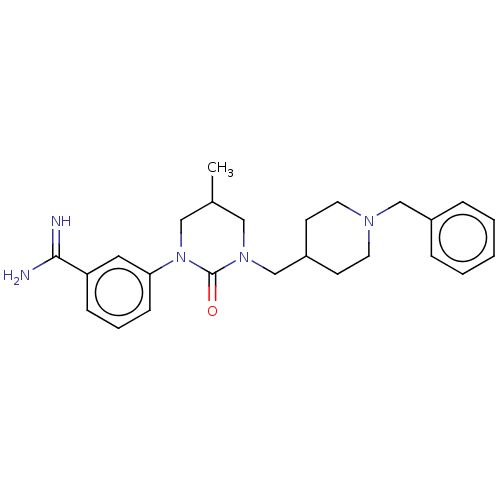 (CHEMBL3781349) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158150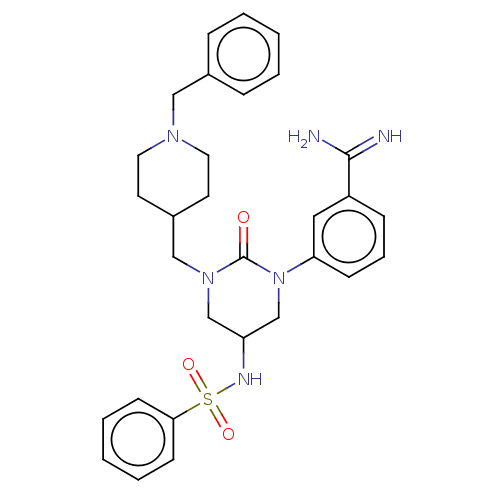 (CHEMBL3780005) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50069105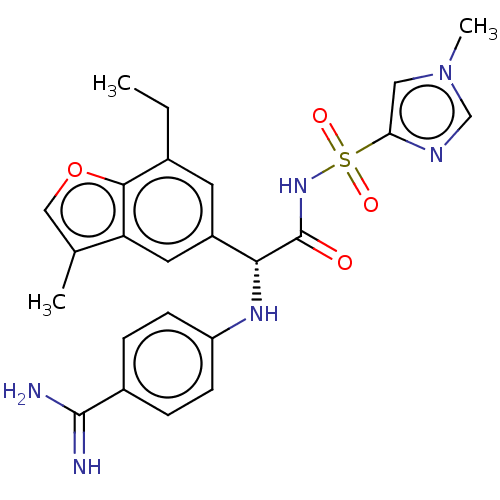 (CHEMBL3403520) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA (unknown origin) after 40 mins by Eadie-Hofstee plot analysis | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158154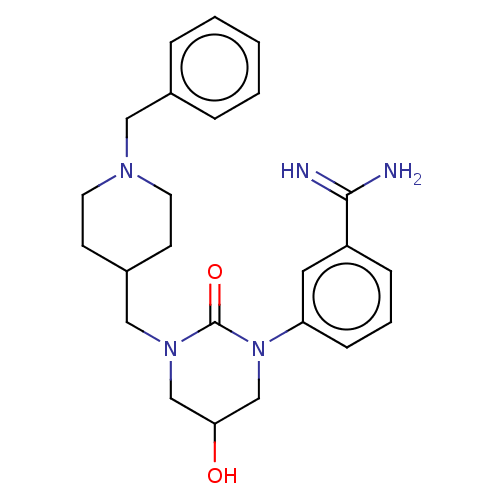 (CHEMBL3781784) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518592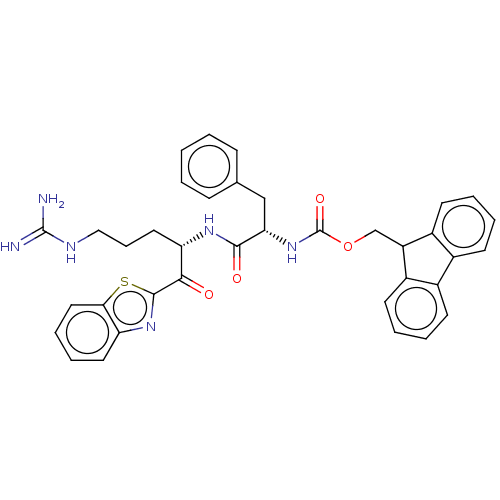 (CHEMBL4528632) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 963 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158152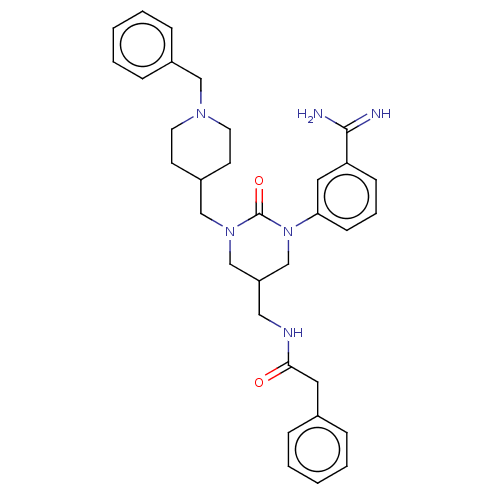 (CHEMBL3780269) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158161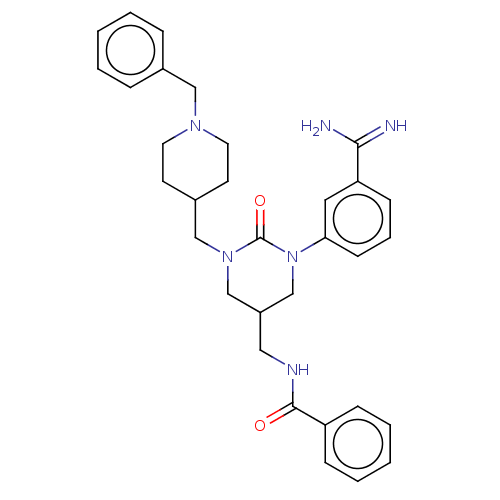 (CHEMBL3781405) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50157633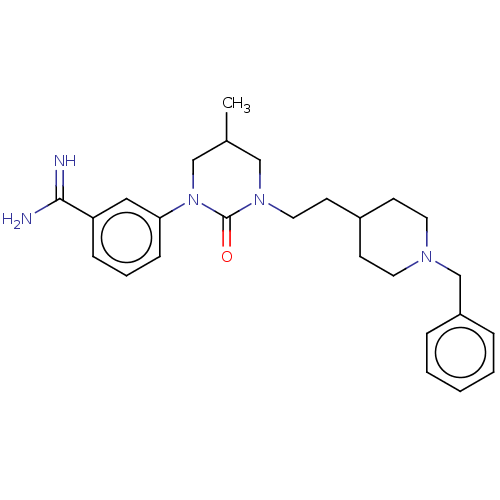 (CHEMBL3779949) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518591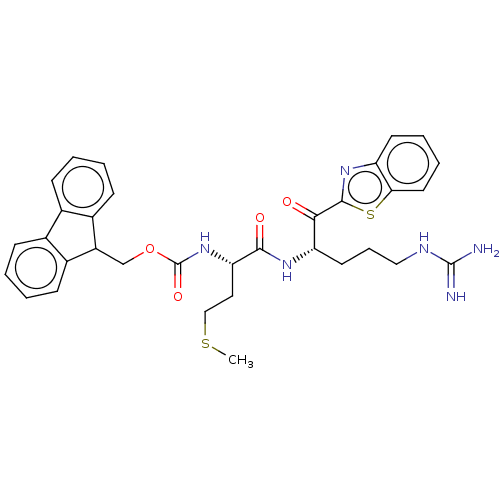 (CHEMBL4552828) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158158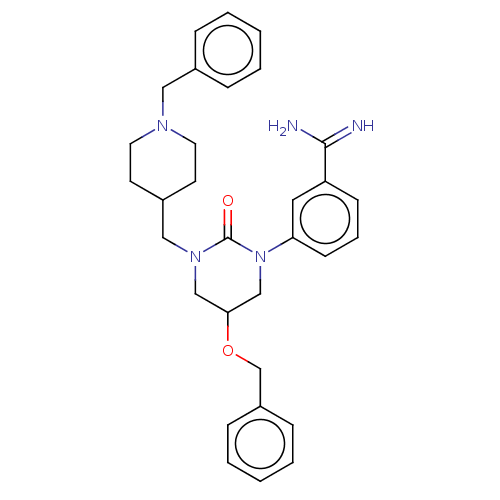 (CHEMBL3780208) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50158156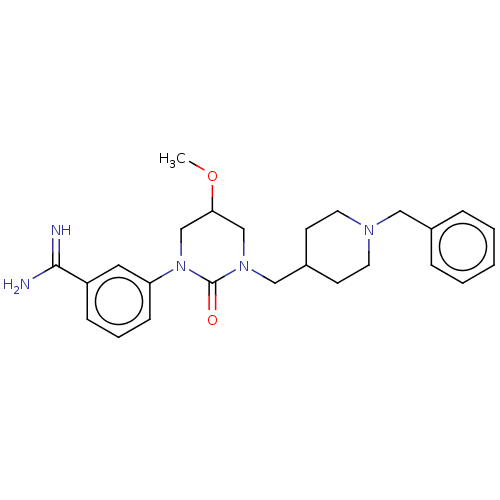 (CHEMBL3781626) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Curated by ChEMBL | Assay Description Inhibition of human recombinant hepatocyte growth factor activator using H2N(EEdansyl)GKQLRVVNGG (KDabcyl)-NH2 as substrate by fluorometric analysis | ACS Med Chem Lett 7: 177-81 (2016) Article DOI: 10.1021/acsmedchemlett.5b00357 BindingDB Entry DOI: 10.7270/Q2Z60QZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518601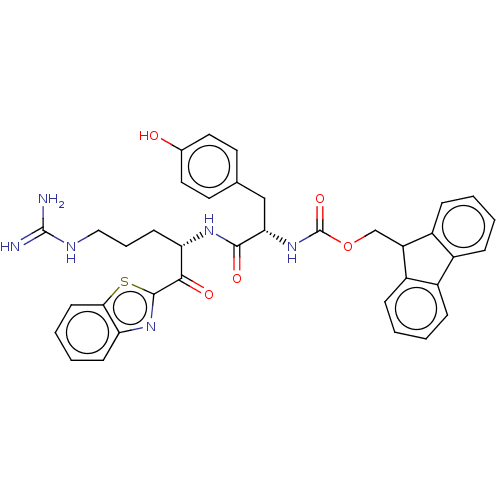 (CHEMBL4460256) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518610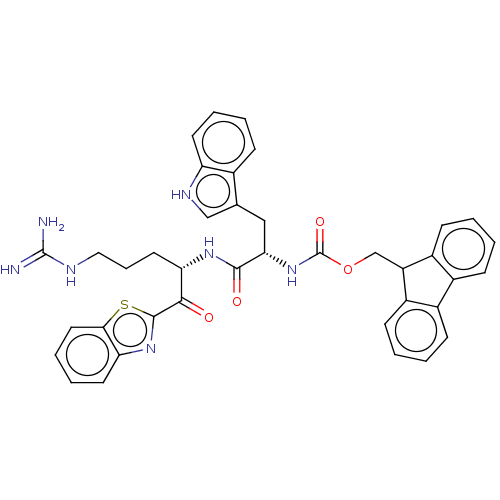 (CHEMBL4528379) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518594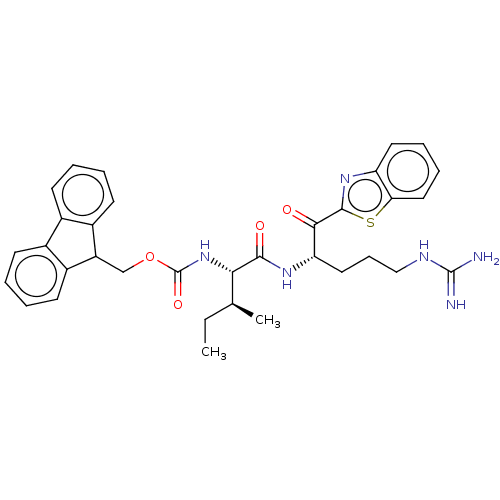 (CHEMBL4445232) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518595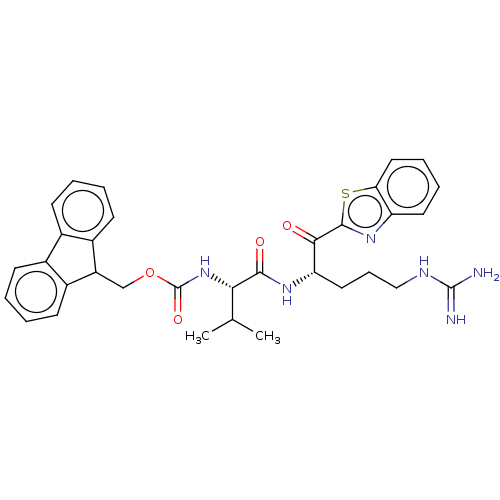 (CHEMBL4472547) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518596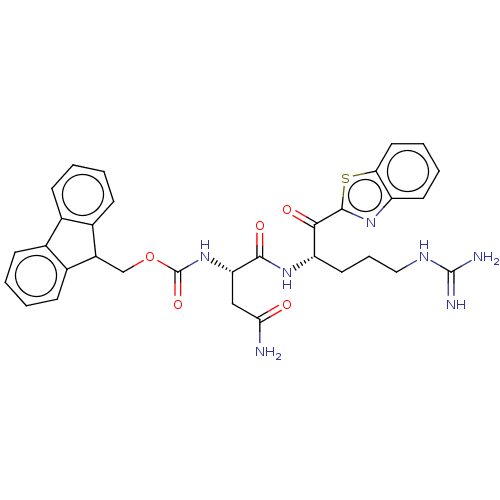 (CHEMBL4463174) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM23886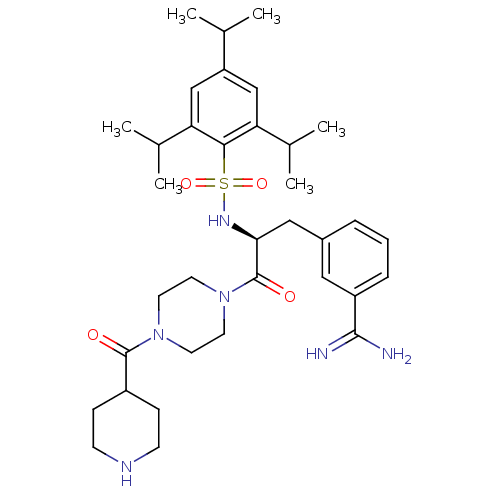 (3-[(2S)-3-oxo-3-[4-(piperidin-4-ylcarbonyl)piperaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal His-tagged HGFA (unknown origin) expressed in baculovirus-infected Sf9 cells using Boc-QLR-AMC as substrate incu... | Bioorg Med Chem 23: 2328-43 (2015) Article DOI: 10.1016/j.bmc.2015.03.072 BindingDB Entry DOI: 10.7270/Q23F4RB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor activator (Homo sapiens (Human)) | BDBM50518604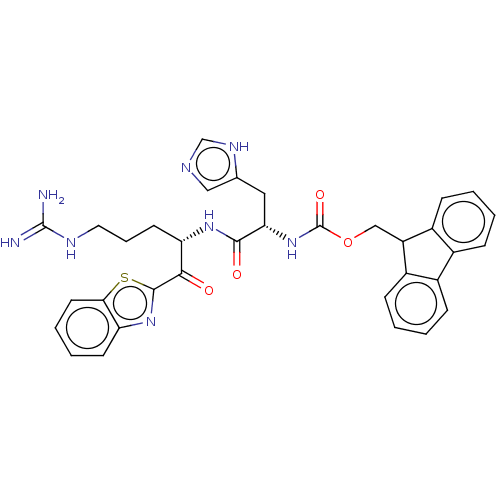 (CHEMBL4551624) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of HGFA catalytic domain (unknown origin) preincubated for 30 mins followed by Boc-QLR-AMC substrate addition and measured for 1 hr by flu... | J Med Chem 62: 480-490 (2019) Article DOI: 10.1021/acs.jmedchem.8b01536 BindingDB Entry DOI: 10.7270/Q2D50RBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79 total ) | Next | Last >> |