

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UDP-glucuronosyltransferase 1A3 (Homo sapiens (Human)) | BDBM50044561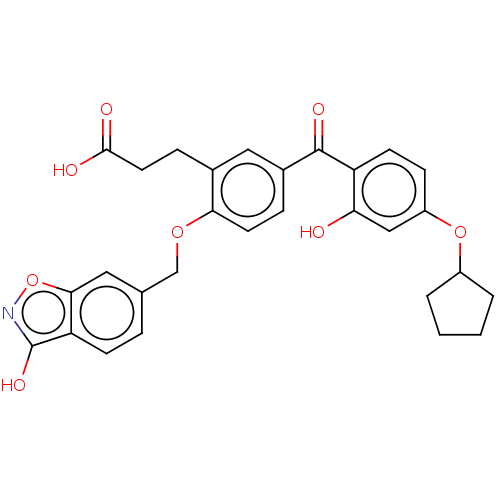 (CHEMBL3222137) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Substrate inhibition of human UGT1A3-mediated T-5224 hydroxyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC ... | Drug Metab Dispos 39: 803-13 (2011) Article DOI: 10.1124/dmd.110.037952 BindingDB Entry DOI: 10.7270/Q2W37Z2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucuronosyltransferase 1A3 (Homo sapiens (Human)) | BDBM50044561 (CHEMBL3222137) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Substrate inhibition of human UGT1A3-mediated T-5224 acyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC meth... | Drug Metab Dispos 39: 803-13 (2011) Article DOI: 10.1124/dmd.110.037952 BindingDB Entry DOI: 10.7270/Q2W37Z2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||