

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM335495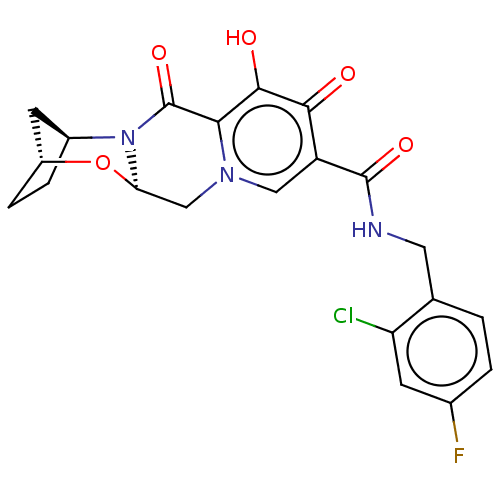 ((2R,5S,13aR)—N-(2-chloro-4-fluorobenzyl)-8-hy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330054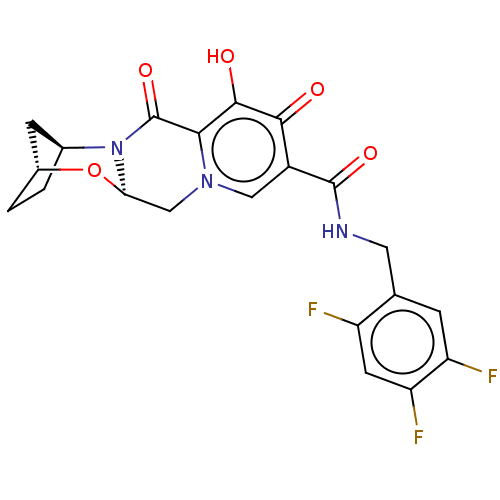 ((2R,5S,13aR)-8-hydroxy-7,9-dioxo-N-(2,4,5-trifluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330049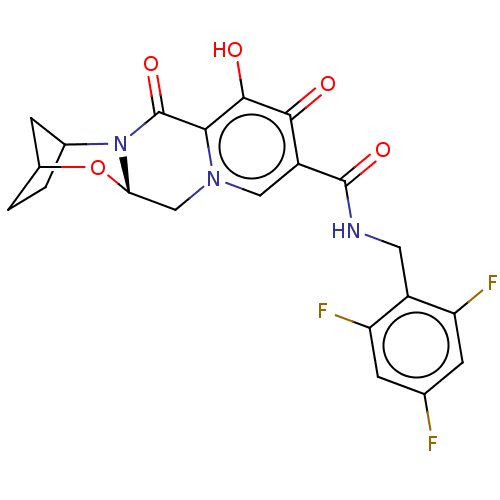 ((13aS)-8-hydroxy-7,9-dioxo-N-(2,4,6-trifluorobenzy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM448201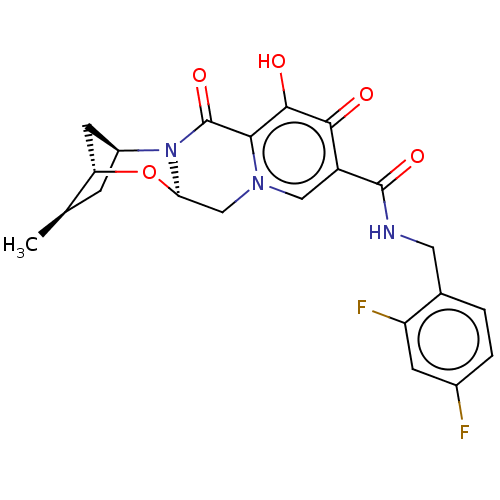 (US10689399, Compound 40) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM350693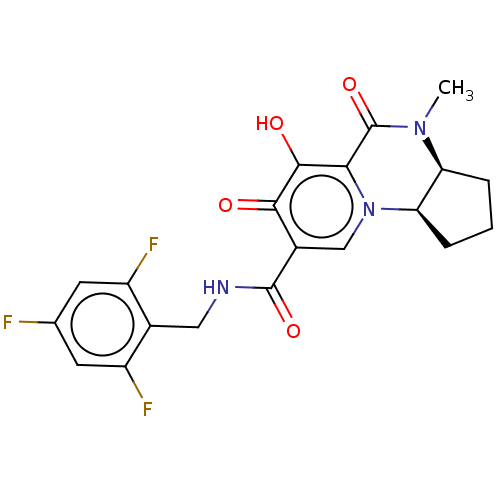 (US9795602, Compound 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
GILEAD SCIENCES, INC. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US9795602 (2017) BindingDB Entry DOI: 10.7270/Q2K35WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330039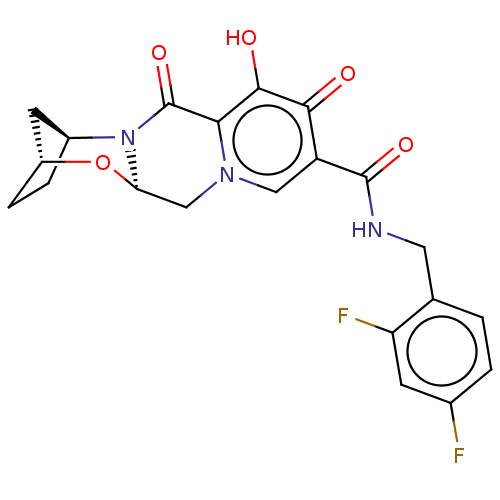 ((2R,5S,13aR)-N-(2,4-difluorobenzyl)-8-hydroxy-7,9-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330040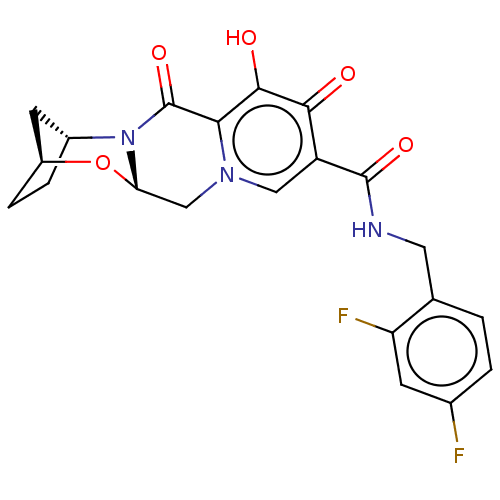 ((2S,5R,13aS)-N-(2,4-difluorobenzyl)-8-hydroxy-7,9-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM448200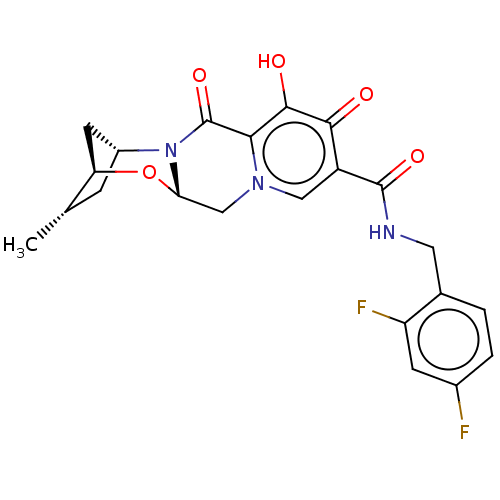 (US10689399, Compound 39) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 358 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM448216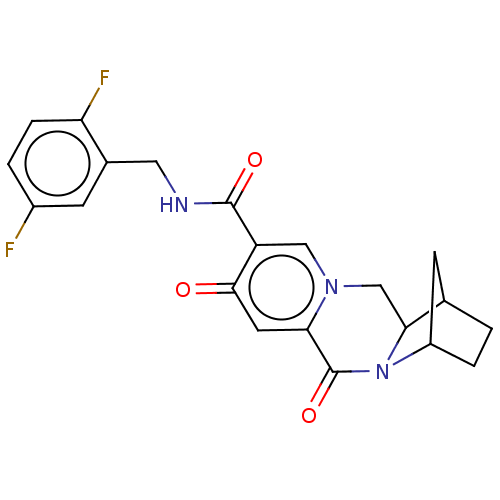 (US10689399, Compound 100) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330052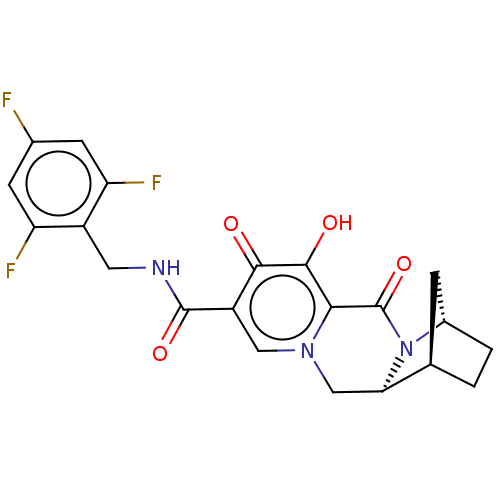 ((1R,4S,12aS)-7-hydroxy-6,8-dioxo-N-(2,4,6-trifluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 476 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330048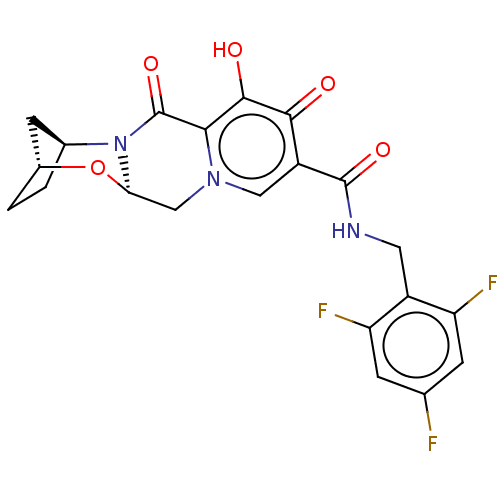 ((2R,5S,13aR)-8-hydroxy-7,9-dioxo-N-(2,4,6-trifluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 487 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM448198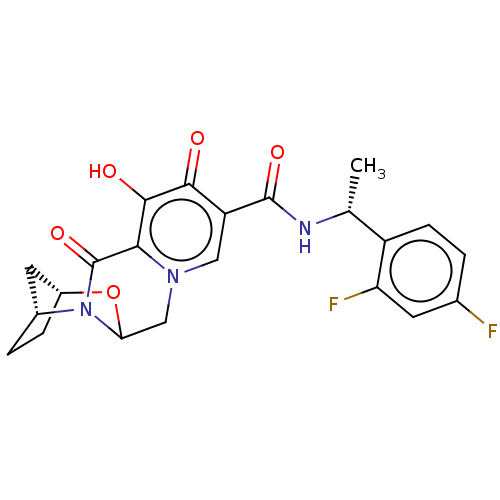 (US10689399, Compound 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM448213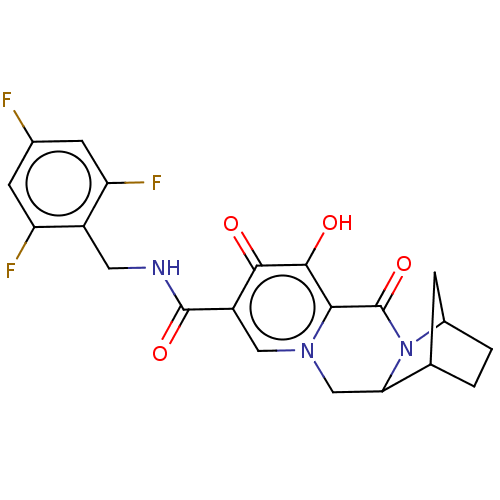 (US10689399, Compound 96) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330060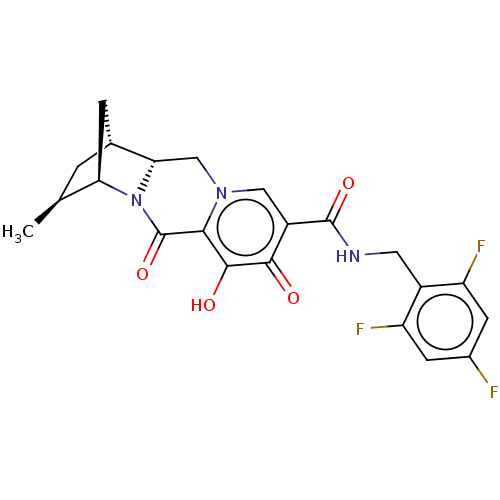 ((1R,3S,4R,12aR)-7-hydroxy-3-methyl-6,8-dioxo-N-(2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330041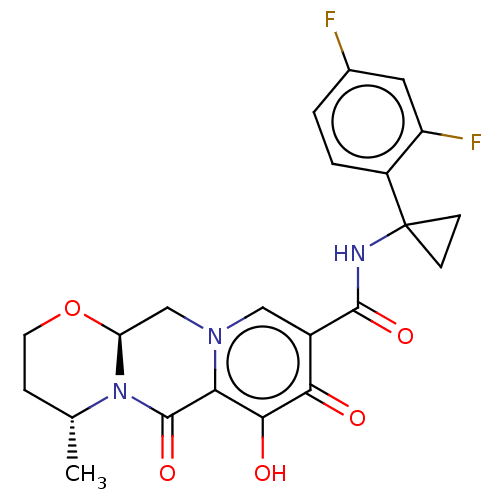 ( (4R,12aS)-N-(1-(2,4-difluorophenyl)cyclopropyl)-7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM350694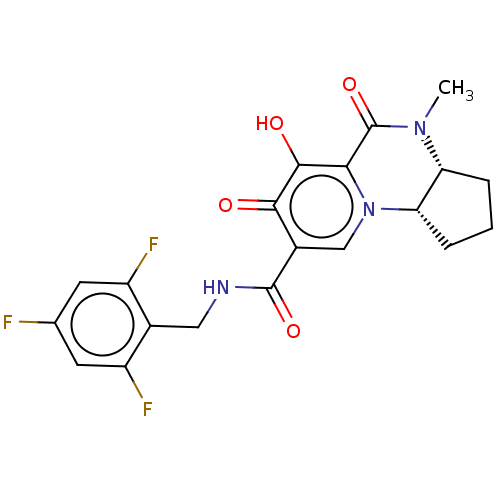 (US9795602, Compound 58) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GILEAD SCIENCES, INC. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US9795602 (2017) BindingDB Entry DOI: 10.7270/Q2K35WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM350830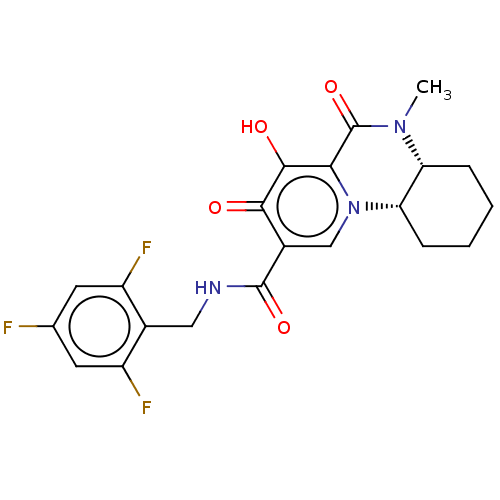 (US9795602, Compound 59) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GILEAD SCIENCES, INC. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US9795602 (2017) BindingDB Entry DOI: 10.7270/Q2K35WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330047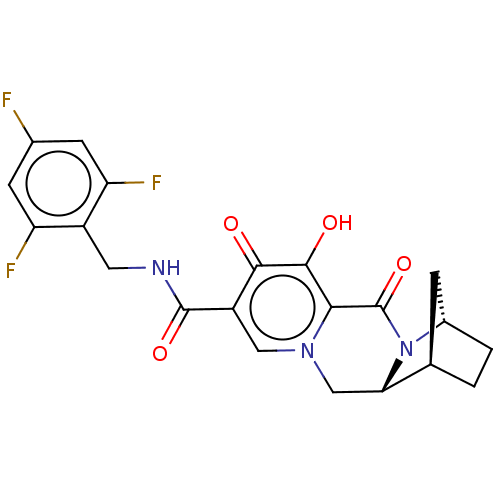 ((1R,4S,12aR)-7-hydroxy-6,8-dioxo-N-(2,4,6-trifluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330059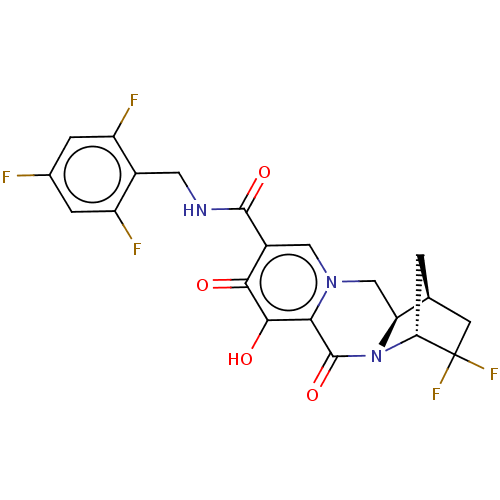 ((1S,4R,12aR)-3,3-difluoro-7-hydroxy-6,8-dioxo-N-(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330055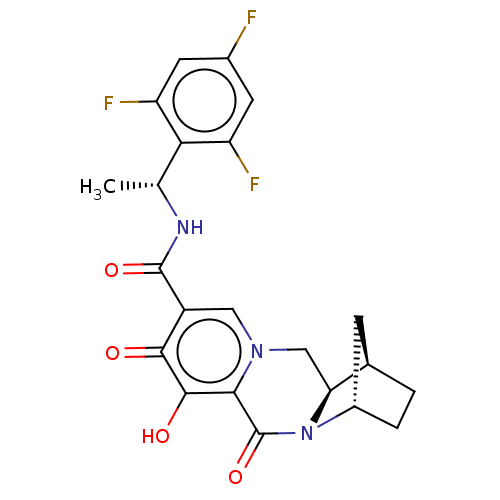 ((1R,4S,12aR)-7-hydroxy-6,8-dioxo-N—((R)-1-(2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330051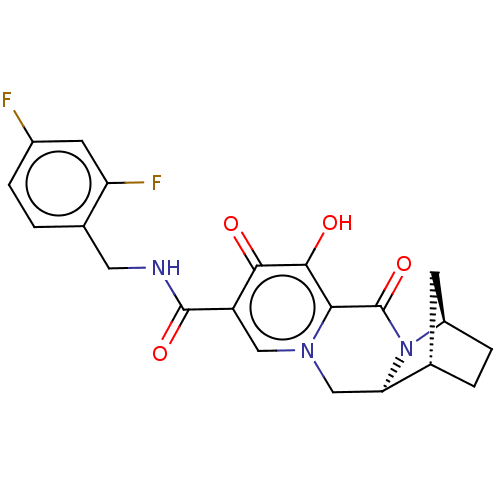 ((1S,4R,12aS)-N-(2,4-difluorobenzyl)-7-hydroxy-6,8-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330050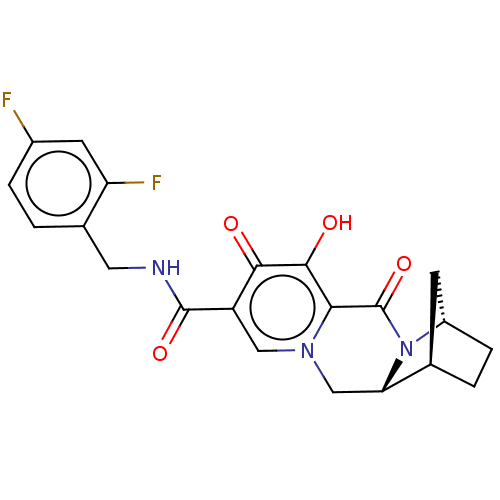 ((1R,4S,12aR)-N-(2,4-difluorobenzyl)-7-hydroxy-6,8-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM350834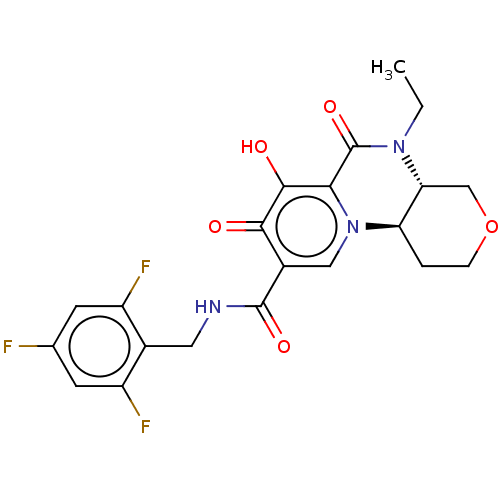 (US9795602, Compound 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GILEAD SCIENCES, INC. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US9795602 (2017) BindingDB Entry DOI: 10.7270/Q2K35WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330057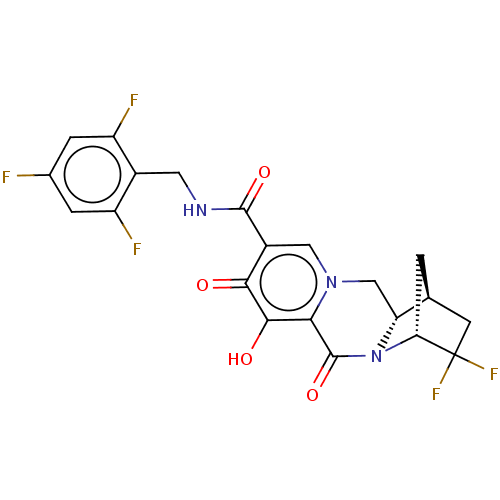 ((1S,4R,12aS)-3,3-difluoro-7-hydroxy-6,8-dioxo-N-(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330056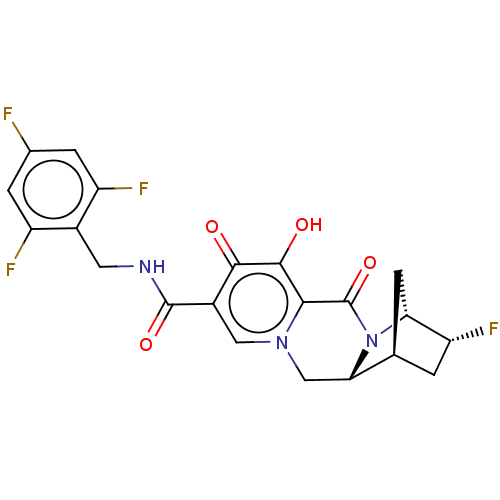 ((1S,3R,4R,12aR)-3-fluoro-7-hydroxy-6,8-dioxo-N-(2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM350831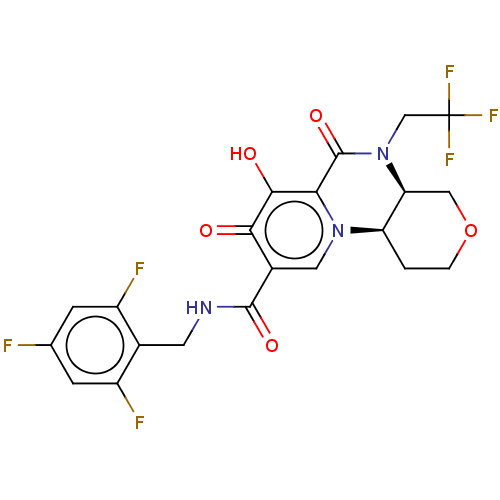 (US9795602, Compound 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GILEAD SCIENCES, INC. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US9795602 (2017) BindingDB Entry DOI: 10.7270/Q2K35WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM350832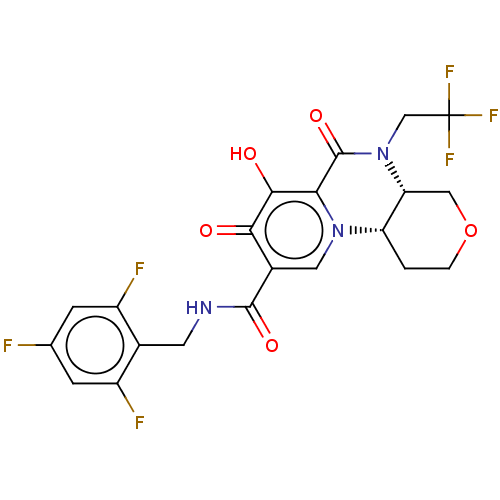 (US9795602, Compound 32) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GILEAD SCIENCES, INC. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US9795602 (2017) BindingDB Entry DOI: 10.7270/Q2K35WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM350833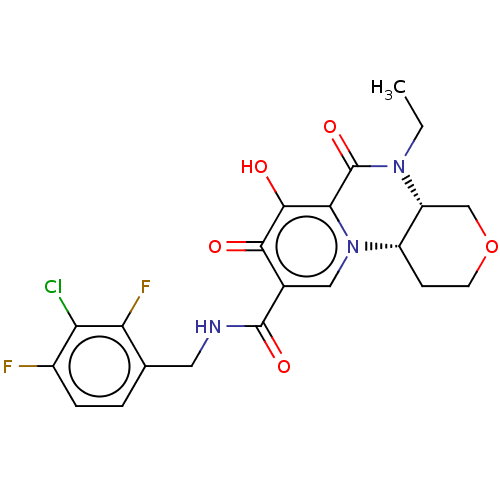 (US9795602, Compound 39) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GILEAD SCIENCES, INC. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US9795602 (2017) BindingDB Entry DOI: 10.7270/Q2K35WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330044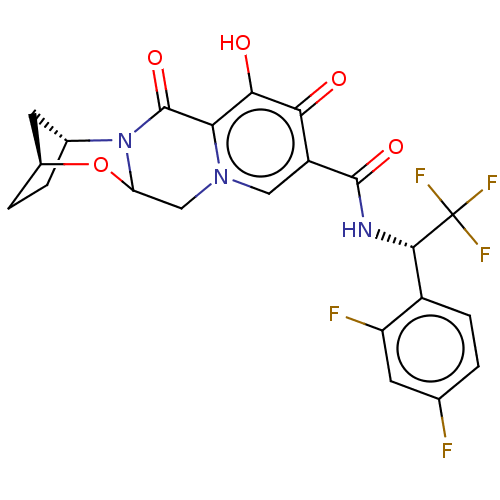 ( (2S,5R)-N—((S)-1-(2,4-difluorophenyl)-2,2,2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330042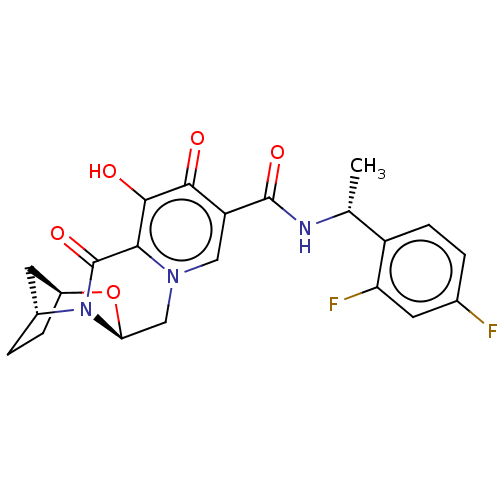 ((2S,5R,13aS)-N—((R)-1-(2,4-difluorophenyl)eth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) BindingDB Entry DOI: 10.7270/Q2S185HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM350836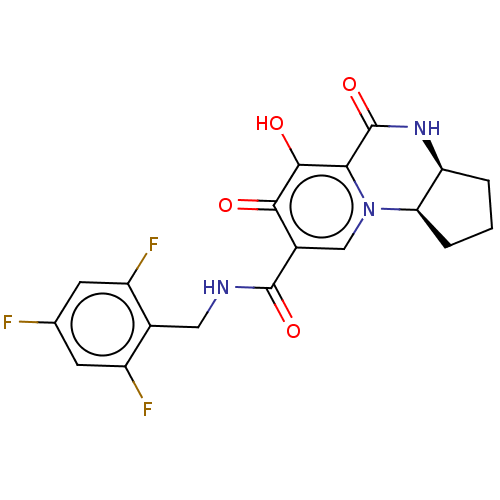 (US9795602, Compound 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GILEAD SCIENCES, INC. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US9795602 (2017) BindingDB Entry DOI: 10.7270/Q2K35WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM350835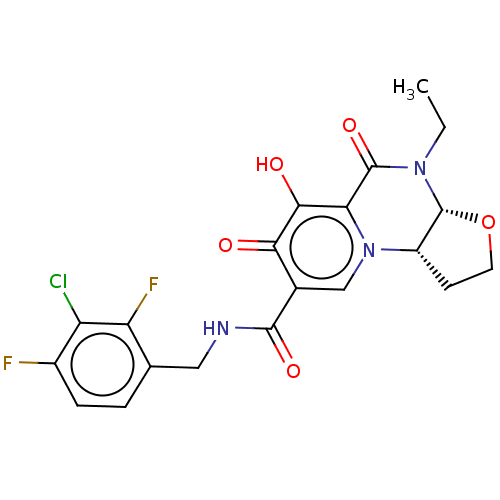 (US9795602, Compound 25b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GILEAD SCIENCES, INC. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US9795602 (2017) BindingDB Entry DOI: 10.7270/Q2K35WTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM50576849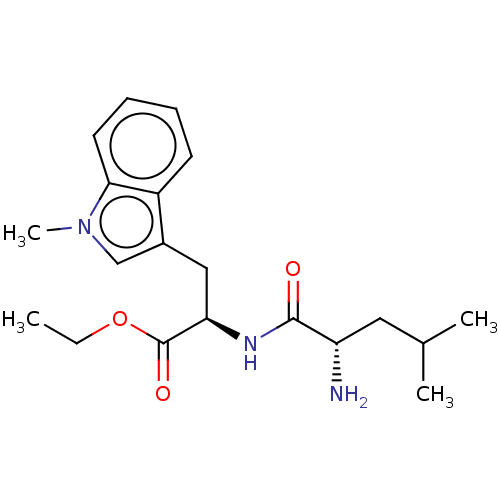 (CHEMBL4876008) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of OCT2 (unknown origin) expressed in HEK293 cells | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112373 BindingDB Entry DOI: 10.7270/Q2XP78Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Boehringer Ingelheim Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of OCT2 (unknown origin) expressed in HEK293 cells assessed as reduction of [14C]metformin substrate uptake at 100 uM by liquid scintillat... | Drug Metab Dispos 41: 149-58 (2012) Article DOI: 10.1124/dmd.112.048470 BindingDB Entry DOI: 10.7270/Q2QC0574 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||